209x Filetype PPTX File size 0.37 MB Source: www.cabarrus.k12.nc.us
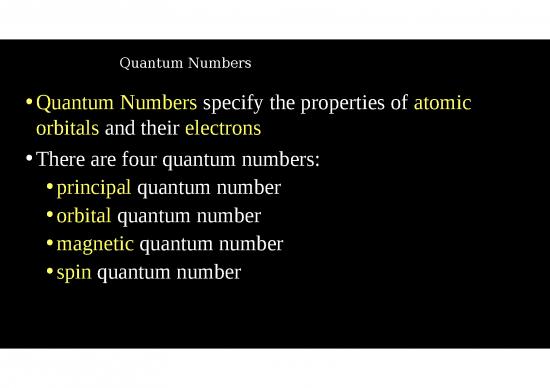
Principal Quantum Number
•The principal quantum number (n) specifies the main energy
levels around the nucleus
•As n increases, the distance from the nucleus increases (So as
the numbers become larger the levels become further from the
nucleus)
•Currently the values for n are 1 to 7
•These orbitals are called subshells or sublevels
•The four most common Principal quantum numbers are given
letter abbreviations
s, p, d, f
Orbital Quantum Number
•Orbital Quantum Number ( l ) indicates
the shape of the orbital where the
electron can be found
“s” Orbital
“p” Orbital
“d” Orbital
no reviews yet
Please Login to review.