203x Filetype PPTX File size 0.32 MB Source: www.lcsnc.org
Think of some properties of salt
• Forms crystals
• Brittle
• Hard
• Solid
• High melting and boiling point
• Forms an electrolyte (conducts
electricity in water)
Ionic Compounds
• NaCl or table salt is an ionic
compound.
• Ionic compounds form from ionic
bonds – bonds formed by
electrostatic attraction of positive
and negative ions
• Positive ions are called cations
• Negative ions are called anions
• Another frequently used description
for ionic bonds is that they form
when electrons are transferred
Ionic Compounds
• Form between metals and nonmetals
• Or between metals and polyatomic
ions (or ammonium and nonmetals).
–A polyatomic ion is a covalently bonded
compound that has lost or gained
electron(s).
The polyatomic ion acts as a unit – one
thing. Never change any part of a
polyatomic ion. If you change any
subscripts you change the entire ion.
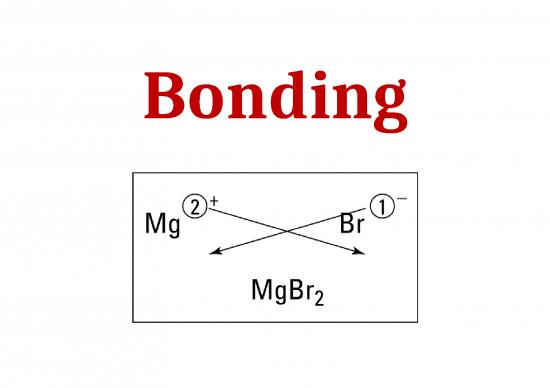
Determining ionic compound formulas
1. Write the element symbols involved in bonding
K O
2. Next, write the oxidation numbers above the symbols
+ 1 - 2
K O
3. Now, move the numbers (only the numbers) across to
the other symbol’s lower right corner – they become the
subscripts
+ 1 - 2
K O
KO
2
Compound formulas with polyatomic ions
+ 2 - 3
Ca PO4
+ 2 - 3
Ca PO4
Ca (PO )
3 4 2
You must use parentheses around
polyatomic ions if there is more than
one of them
no reviews yet
Please Login to review.