263x Filetype PDF File size 1.50 MB Source: www.csus.edu
PolarPolar CCovalentovalent Bonds:Bonds: ElectronegativityElectronegativity
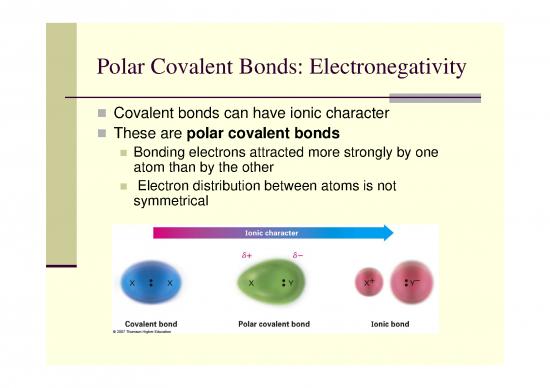
Covalent bonds can have ionic character
These are polar covalent bonds
Bonding electrons attracted more strongly by one
atomatom thanthan byby thethe ootherther
Electron distribution between atoms is not
symmetrical
BondBond PolarityPolarity andand ElectronegativityElectronegativity
Symmetrical Covalent Bonds Polar Covalent Bonds
C – C + -
C – H C – O
(non-polar)
(polar)
Electronegativity (EN): intrinsic ability of an atom to
attractattract tthehe sharedshared electronselectrons inin aa ccovalentovalent bondbond
Inductive Effect: shifting of sigma bonded electrons
in resppyonse to nearby electroneggative atom
TheThe PPeriodiceriodic TTableable andand ElectronegativityElectronegativity
C – H C - Br and C - I
((non-pol)lar) ((pollar))
BondBond PolarityPolarity andand IInductivenductive EEffectffect
Nonppolar Covalent Bonds: atoms with similar EN
Polar Covalent Bonds: Difference in EN of atoms <
2
Ionic Bonds: Difference in EN > 2
C–H bonds, relatively nonpolar C-O, C-X bonds
((moremore electronegativeelectronegative elements)elements) aarere polarpolar
Bonding electrons shift toward electronegative atom
C acqqppuires partial positive chargge,, +
Electronegative atom acquires partial negative
charge, -
Inductive effect: shifting of electrons in a bond in
response to EN of nearby atoms
no reviews yet
Please Login to review.