226x Filetype PPTX File size 0.34 MB Source: brannel.com
Diamond Metallic bonding Metallic bonding
Number of covalent bonds from each Describe metallic bonding
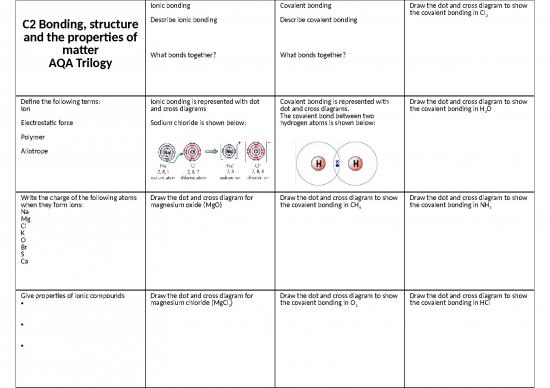
C2 Bonding, structure carbon
and the properties of Melting point is low / high / very high
matter Why doesn’t it conduct What bonds together?
electricity?
AQA Trilogy
There are several ways to represent Graphite Why are most metals: Draw a diagram to show why alloys are
covalent bonds: Number of covalent bonds from each solid at room temperature? harder than pure metals
carbon
Melting point is low / high / very high
Good conductors of electricity and heat
Why does it conduct electricity?
The repeating unit of poly(ethene) is Write about the uses of Draw a diagram to show why most
shown below. What is the molecular Fullerenes like Bucky balls metals are malleable
formula of poly(ethene) and nanotubes
Predict the state of:
o
Bromine at room temperature (25 C)
Nitrogen at room temperature (25oC)
o
Oxygen at – 220 C
o
The reason that most polymers are solid Name the process: Draw particle diagrams to show a solid, Ethanol melts at -114 C and boils at
o
at room temperature is: Solid liquid liquid and a gas 78 C. Predict the state at:
Liquid gas o
Gas liquid -150 C
solid liquid gas
Liquid solid o
0 C
o
25 C
o
100 C
no reviews yet
Please Login to review.