264x Filetype XLSX File size 0.10 MB Source: effectivehealthcare.ahrq.gov
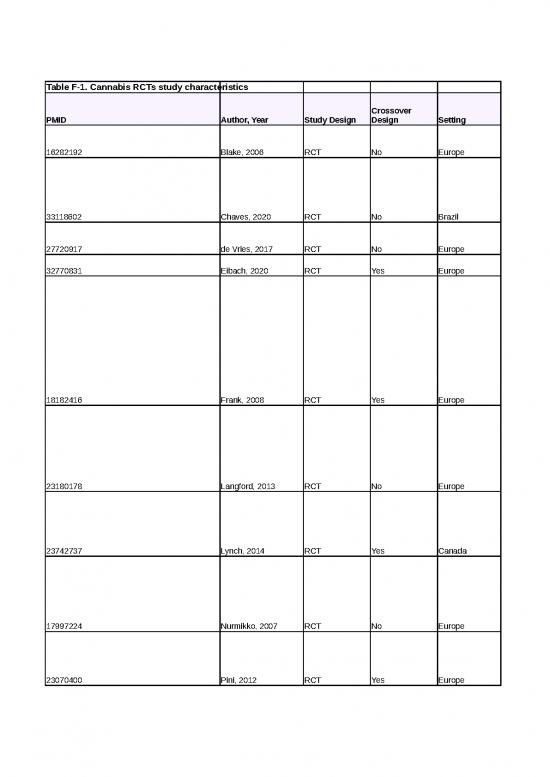
Table F-1. Cannabis RCTs study characteristics
Crossover
PMID Author, Year Study Design Design Setting
16282192 Blake, 2006 RCT No Europe
33118602 Chaves, 2020 RCT No Brazil
27720917 de Vries, 2017 RCT No Europe
32770831 Eibach, 2020 RCT Yes Europe
18182416 Frank, 2008 RCT Yes Europe
23180178 Langford, 2013 RCT No Europe
23742737 Lynch, 2014 RCT Yes Canada
17997224 Nurmikko, 2007 RCT No Europe
23070400 Pini, 2012 RCT Yes Europe
20855984 Rintala, 2010 RCT Yes U.S.
16186518 Rog, 2005 RCT No Europe
29073592 Schimrigk, 2017 RCT No Europe
19808912 Selvarajah, 2010 RCT No Europe
24420962 Serpell, 2014 RCT No Europe
17974490 Skrabek, 2008 RCT No Canada
22921260 Toth, 2012 RCT No Canada
25288189 Turcotte, 2015 RCT No Canada
16988792 Wissel, 2006 RCT Yes Germany
31793418 Xu, 2020 RCT No U.S.
22791906 Zajicek, 2012 RCT No Europe
Abbreviations: CBD = cannabidiol; HIV = human
immunodeficiency virus; NA = not applicable;
NPP = neuropathic pain; NR = not reported;
RCT = randomized controlled trial; THC =
tetrahydrocannabinol; U.S. = United States
Age Measure
Pain Population Pain Condition (Mean, Median) Age, Years Female % Race, White %
Inflammatory arthritis Rheumatoid arthritis Mean 63 79 NR
Fibromyalgia Fibromyalgia Mean 52 100 NR
Chronic pancreatitis and
postsurgical abdominal
Visceral pain pain Mean 53 50 96
NPP HIV-associated Mean 50 3 NR
NPP NR Mean 50 23 NR
NPP Multiple sclerosis Mean 49 68 98
Chemotherapy-induced
NPP neuropathic pain Mean 56 83 NR
NPP Mixed Mean 53 59 NR
Medication overuse
Headache headache Mean 53 67 NR
no reviews yet
Please Login to review.