265x Filetype XLSX File size 0.10 MB Source: www.anses.fr
Sheet 1: Tableau_valide
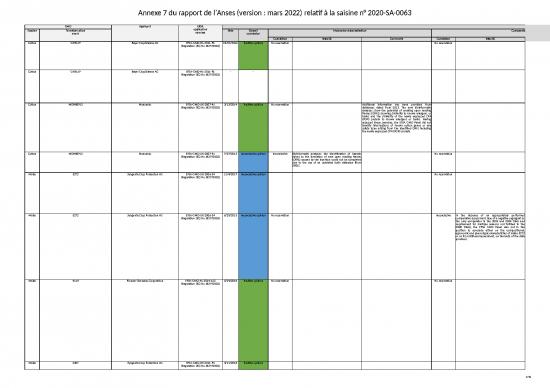
| GMO | Applicant | EFSA application number |
EFSA opinion | ||||||||||||||||||||||||||||||||||
| Species | Transformation event |
Date | Overall conclusion |
Molecular characterisation | Comparative analysis | Toxicology | Allergenicity | Nutritional assessment | Date | Overall conclusion |
Molecular characterisation | Comparative analysis | Toxicology | Allergenicity | Nutritional assessment | ||||||||||||||||||||||
| Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | Conclusion | Issue(s) | Comments | ||||||||
| Cotton | GHB119 | Bayer CropScience AG | EFSA-GMO-NL-2011-96 (Regulation (EC) No 1829/2003) |
10/21/2016 | Positive opinion | No reservation | No reservation | The GMO Panel considered the lower crude protein content and total tocopherol levels in cotton GHB119 of no safety concern, since a proper diet formulation can balance these endpoints. | No reservation | The GMO Panel did not consider the 90-day study in its evaluation, because the cage should be considered the experimental unit and thus the number of experimental units per treatment (two per sex) is too low. | No reservation | No reservation | 11/30/2016 | Negative opinion | No reservation | The « Biotechnology » working group underlines that the prediction of the expression of the new ORFs by in silico analyses has limitations and proposes to complete this approach with a study of the transcription of the region where the T-DNA was integrated. | Reservations | New statistical analyses were provided for the 2007 and 2008 trials in Spain, but although they were carried out in 2012 and 2016, they were not performed according to the EFSA (2010) recommendations. Additionally, the equivalence of cotton GHB119 with conventional cotton varieties isn't demonstrated. | The reservation about the control used in the analysis of the derived products is lifted. | Reservations | The reservations expressed in the 2012 opinion about the 90-day study are maintained, except the one concerning the control. The power analysis was not performed whereas the experimental data are adequate to perform such an analysis. | The reservation related to the use of GHB119 in the 90-day study is lifted. | No reservation | The results of the updated bioinformatic analyses (2013, 2015 and 2016) don't change the conclusions of the 2012 opinion regarding the allergenicity assessment. | Reservations | The reservations expressed in the 2012 opinion are maintained. | |||||||||||
| Cotton | GHB119 | Bayer CropScience AG | EFSA-GMO-NL-2011-96 (Regulation (EC) No 1829/2003) |
- | - | 2/4/2012 | Negative opinion | No reservation | Reservations | The analysis of the derived products was performed with a control that is not the isogenic line. In the compositional analysis of the whole grain, the equivalence tests as recommended by EFSA (2010) were not performed. The compositional analysis of the meal doesn't allow to conclude to the absence of difference between cotton GHB119 and the control. | Reservations | 90-day trial not admissible: uncertainty about the use of cotton GHB199 and about the fact that the plants were sprayed or not with glufosinate. | No reservation | Reservations | Non admissible poultry trial: unjustified very low incorporation rate of the meal in the poultry diets and high mortality in all the groups. | ||||||||||||||||||||||
| Cotton | MON88913 | Monsanto | EFSA-GMO-UK-2007-41 (Regulation (EC) No 1829/2003) |
3/13/2014 | Positive opinion | No reservation | Additional information has been provided from databases dated from 2013. The new bioinformatic analyses show the potential of creating open reading frames (ORFs) showing similarity to known allergens or toxins and the similarity of the newly expressed CP4 EPSPS protein to known allergens or toxins. Having assessed these searches, the EFSA GMO Panel did not identify interruptions of known cotton genes or any safety issue arising from the identified ORFs including the newly expressed CP4 EPSPS protein. | - | - | ||||||||||||||||||||||||||||
| Cotton | MON88913 | Monsanto | EFSA-GMO-UK-2007-41 (Regulation (EC) No 1829/2003) |
7/29/2013 | Inconclusive opinion | Inconclusive | Bioinformatic analyses: the identification of hazards linked to the formation of new open reading frames (ORFs) caused by the insertion could not be completed due to the use of an outdated toxin database (from 2001). | No reservation | No reservation | No reservation | No reservation | 1/18/2008 | Positive opinion | No reservation | No remark concerning the obsolescence of one of the databases used for the bioinformatic analyses. | No reservation | No reservation | No reservation | Current knowledge doesn't make it possible to completely ensure non-allergenicity of peptides resulting from the digestion of the protein. The comment only underlines the front of science. | No reservation | |||||||||||||||||
| Maize | 3272 | Syngenta Crop Protection AG | EFSA-GMO-UK-2006-34 (Regulation (EC) No 1829/2003) |
11/4/2019 | Inconclusive opinion | No reservation | None of the differences identified in forage and grain composition between maize 3272 and its conventional counterpart needs further assessment regarding food and feed safety, except for higher ferulic acid levels in grains of maize 3272 that is reported in Section 3.3.2. | Inconclusive | Regarding the allergenic potential of AMY797E protein and considering all possible food and feed uses of maize 3272, the Panel concludes that the information provided does not fully address the concerns previously raised in 2013. Owing to the nature and the knowledge available on this protein family, it is still unclear whether under specific circumstances the alpha-amylase AMY797E has the capacity to sensitise certain individuals and to cause adverse effects. | The applicant provided thorough information relevant for the allergenicity assessment of dried distiller grains with solubles (DDGS), which is the main product of interest for importation into the EU. Having considered the information provided on this product, the Panel is of the opinion that under the specific conditions of use described by the applicant, DDGS produced from maize 3272 does not raise concerns when compared to DDGS from non-GM maize. | 1/30/2020 | Negative opinion | No reservation | No reservation | Reservations | Insufficient number of rats in the 28-day toxicity study performed on the AMY797E protein. No 28-day toxicity study for the PMI protein. Weaknesses in the 90-day study: presence of maize 3272 in the control, the low dose diets are not complemented with non-GM maize to reach the same maize percentage as that of the high dose diets, absence of commercial varieties, insufficient number of animals/group/sex and absence of power analysis. | Reservations | Due to its resistance to thermal denaturation and its high concentration in mature grains, the question of an allergenic potential of the AMY797E protein is raised in case of an ingestion other than sporadic. In the context of an occupational exposure, alpha-amylase is an allergen that has been incriminated in cases of sensitisation by inhalation and skin contact. | Reservations | The presence of measurable levels of the AMY797E protein in grains and diets of the control raises questions about the validity of the feeding study in broiler chickens. | |||||||||||||||||
| Maize | 3272 | Syngenta Crop Protection AG | EFSA-GMO-UK-2006-34 (Regulation (EC) No 1829/2003) |
6/20/2013 | Inconclusive opinion | No reservation | Inconclusive | In the absence of an appropriately performed comparative assessment (use of a negative segregant as the only comparator in the 2003 and 2004 trials and requirement for multiple seasons not fulfilled in the 2008 trials), the EFSA GMO Panel was not in the position to conclude either on the compositional, agronomic and phenotypic characteristics of maize 3272 or on its nutritional assessment, on the basis of the data provided. | Inconclusive | The safety assessment could not be completed in the absence of an appropriately performed comparative assessment. The EFSA GMO Panel did not consider the repeated-dose 90-day oral toxicity study and the feeding study in broiler chickens, because the material derived from a negative segregant administered as the control material is not adequate for the safety assessment of food/feed from GM plants. | The safety assessment has focused mainly on the newly expressed proteins. No indications of safety concern over the toxicity of the AMY797E and PMI proteins were identified. | Inconclusive | The Panel could not conclude on the potential for de novo allergic sensitisation of the AMY797E protein. | Inconclusive | In the absence of an appropriately performed comparative assessment (use of a negative segregant as the only comparator in the 2003 and 2004 trials and requirement for multiple seasons not fulfilled in the 2008 trials), the EFSA GMO Panel was not in the position to conclude either on the compositional, agronomic and phenotypic characteristics of maize 3272 or on its nutritional assessment, on the basis of the data provided. The EFSA GMO Panel did not consider the repeated-dose 90-day oral toxicity study and the feeding study in broiler chickens, because the material derived from a negative segregant administered as the control material is not adequate for the safety assessment of food/feed from GM plants. | 9/17/2007 | Reserved opinion | No reservation | Reservations | As no data was provided regarding the concentration and the residual activity of the AMY797E protein in the co-products, it is not possible to check if this protein has been destroyed or if it lost activity in the co-products intended for use as feed. | No reservation | No reservation | Maize 3272 is unlikely to present an allergenic potential linked to the AMY797E and PMI proteins, but a check of the absence of cross reaction of the AMY797E protein with the serum of patients allergic to alpha-parvalbumin would have allowed to strengthen this position. Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | No reservation | |||||||||||||
| Maize | 4114 | Pioneer Overseas Corporation | EFSA-GMO-NL-2014-123 (Regulation (EC) No 1829/2003) |
5/24/2018 | Positive opinion | No reservation | No reservation | No reservation | Only one dose level was tested (32% w/w) in the 90-day test. Renal tubule tumours (in two males fed the test diet 4114 (event 4114 unsprayed with glufosinate)) were observed in the initial repeated-dose 90-day oral toxicity study, but were not confirmed in a second trial carried out with a larger number of animals : the observed adenomas and carcinomas and renal tubule hyperplasias were concluded to be spontaneous lesions unrelated to the consumption of the test diet. Besides these histopathological data in two males, higher mean haemoglobin and haematocrit values were observed in males fed the test diet 4114 (event 4114 unsprayed with glufosinate); as well as significant higher mean alkaline phosphatase (~20%) and creatinine (20%), and lower mean absolute (11%) and relative-to-brain (10%) weights of epididymes in males fed the test diet 4114 GLU (event 4114 sprayed with glufosinate). | No reservation | No reservation | 8/6/2015 | Positive opinion | No reservation | No reservation | Products derived from maize 4114 have not been analysed. | No reservation | Renal tumours recorded in two rats fed maize 4114 unsprayed with glufosinate (NT) have not been confirmed in a second trial carried out on two groups of 20 males and 20 females rats fed control and 4114 NT maize. | No reservation | Adjuvant properties were observed in case an anti-acid (pepsin inhibitor) is given at the same time as Cry proteins (high concentration treatment). | No reservation | ||||||||||||||||
| Maize | 5307 | Syngenta Crop Protection AG | EFSA-GMO-DE-2011-95 (Regulation (EC) No 1829/2003) |
4/11/2018 | Positive opinion | No reservation | - | - | |||||||||||||||||||||||||||||
| Maize | 5307 | Syngenta Crop Protection AG | EFSA-GMO-DE-2011-95 (Regulation (EC) No 1829/2003) |
5/5/2015 | Inconclusive opinion | Inconclusive | Relevant similarities between the amino acid sequence of PMI and a known allergen, and between the amino acid sequence of eCry3.1Ab and a potential toxin. | No reservation | Some agronomic and phenotypic differences between maize 5307 and its conventional counterpart (higher ‘heat units to 50 % pollen shed’, grain moisture, plant height, grain yield). | Inconclusive | Inadequate 28-day toxicity study provided for the eCry3.1Ab protein. | Inconclusive | The toxicity study with the eCry3.1Ab protein is considered inadequate. Therefore, the potential adverse effects on the immune system cannot be assessed. | In the context of this application, the EFSA GMO Panel considered that there are no indications that the newly expressed PMI protein in maize 5307 may be allergenic. |
No reservation | Outcome of the broiler feeding study not assessed due to study weaknesses. | 9/20/2011 | Negative opinion | No reservation | Explanations about the interest of protein eCry3.1Ab as compared to mCry3A would have been appreciated. | Reservations | Absence of commercial varieties in some of the field trials. | Reservations | Absence of a repeated-dose 90-day oral toxicity study on whole food/feed in rodents. | The results presented allow to conclude that the newly expressed proteins eCry3.1Ab and PMI are safe. | No reservation | Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | No reservation | |||||||||
| Maize | MON87403 | Monsanto | EFSA-GMO-BE-2015-125 (Regulation (EC) No 1829/2003) |
3/28/2018 | Positive opinion | No reservation | No reservation | The combined-site analysis of 13 field trial sites showed that R1 and R6 ear biomass were higher for maize MON87403 compared to the conventional counterpart. As there was only partial overlap among the sites used for the agronomic, phenotypic and compositional characterisation of maize MON87403 and those used for its physiological characterisation, the GMO Panel verified whether the intended trait, increased R1 ear biomass, was observed in the sites used for the compositional analysis. Based on the provided data, four out of seven sites from which samples were taken for the compositional analysis, phenotypic manifestation of the intended trait was realised. For these sites, the ear biomass (at the R1 or R6 stage) was higher. However, only for one site the increase in ear biomass was statistically significant at the R1 and R6 stages, which raised the question on whether compositional data obtained from the field trials would allow a thorough risk assessment. The GMO Panel acknowledges that the change due to the intended trait is known to be of limited amplitude, and that the AtHB17Δ113 protein is expressed in maize MON87403, which suggests that the manifestation of the trait may depend on environmental conditions in the field trials. The GMO Panel concludes that the agronomic, phenotypic and compositional analysis did not identify issues requiring further assessment regarding food and feed safety and its environmental impact. | No reservation | No reservation | No reservation | 12/22/2015 | Reserved opinion | No reservation | No reservation | On the basis of the provided information, maize MON87403 appears to be equivalent to the reference commercial varieties regarding the grain and forage composition, as well as for the agronomic and phenotypic traits, including yield at harvest. The actual presence of the claimed traits in this maize is therefore not demonstrated. It is surprising that the results of Rice et al. (2014) and Leibman et al. (2014) have not been included in the analysis. | No reservation | No reservation | No reservation | ||||||||||||||||||
| Maize | MON89034 | Monsanto | EFSA-GMO-NL-2007-37 (Regulation (EC) No 1829/2003) |
12/18/2008 | Positive opinion | No reservation | No reservation | No reservation | A numerically higher incidence of kidney alterations in females of the high dose group was attributable to two rats. It seems unlikely that the urinary bladder calculi and associated kidney alterations could have been induced by the tested maize in 14 days. A low incidence of urinary bladder calculi is known to occur in this rat strain and may be considered a spontaneous finding in sub-chronic studies. | No reservation | No reservation | A small but statistically significant difference in adjusted feed conversion (a calculated parameter) in males was observed between broilers fed maize MON89034 and control broilers (1.59 vs 1.64 kg/kg). Compared to three of the four conventional maize varieties, the adjusted feed conversion was not significantly different. | 7/25/2012 | Negative opinion | Reservations | 90-day study: based on the provided information, a link between bladder stones (calculi) and diet cannot be ruled out (significant difference, p < 0.01). | |||||||||||||||||||||
| Maize | MON89034 | Monsanto | EFSA-GMO-NL-2007-37 (Regulation (EC) No 1829/2003) |
- | - | 11/20/2007 | Negative opinion | Reservations | An extension of the sequencing by about 1000 bp on each side of the insertion would be desirable. | No reservation | Reservations | 90-day study: further explanation should be provided for the difference in occurrence of calculi in the bladder between the historical data (0.49%) and the 10% incidence (base 20 animals) observed in female animals in the high dose group of MON89034. | No reservation | No reservation | |||||||||||||||||||||||
| Rapeseed | MON88302 | Monsanto | EFSA-GMO-BE-2011-101 (Regulation (EC) No 1829/2003) |
6/17/2014 | Positive opinion | No reservation | No reservation | Agronomic and phenotypic characteristics differences were observed between MON88302 and its conventional counterpart for days-to-first flowering. This observed difference was further assessed (Section 6). | No reservation | No reservation | No reservation | 6/14/2012 | Negative opinion | Reservations | No information about the expression level of the transgene in the pollen. | Reservations | The conditions in which the compositional analysis was performed are incomplete (no late treatment of the plants). | Reservations | No repeated-dose 90-day oral toxicity study in rodents on whole food/feed. | No reservation | Reservations | No nutritional study. | |||||||||||||||
| Soybean | 305423 | Pioneer | EFSA-GMO-NL-2007-45 (Regulation (EC) No 1829/2003) |
12/18/2013 | Positive opinion | No reservation | No reservation | The composition of soybean 305423 differs from that of the conventional counterpart and of non-GM reference varieties in its fatty acid profile, the newly expressed protein, the minerals zinc and calcium and the isoflavone glycitin. A safety and nutritional assessment of the altered fatty acid profile and the newly expressed protein is provided in section 5 of this Scientific Opinion. For the remaining compounds, no further assessment was deemed necessary owing to their well-known biochemical roles and to the magnitude of the reported levels. No differences were identified in the agronomic and phenotypic characteristics that would require further assessment with regard to safety. | No reservation | A repeated-dose 90-day oral toxicity study, in which material derived from a negative segregant is administered as the sole control material, has limitations for the safety assessment, principally because of an inability to detect unintended effects. An appropriate non-GM genotype with a genetic background as close as possible to soybean 305423 with a history of safe use (conventional counterpart) should have been included in this study. However, three commercial non-GM varieties were included providing information on the normal range. The values and/or nature of the findings were comparable throughout all groups. | No reservation | No reservation | A 42-day feeding study in broilers, in which material derived from a negative segregant is administered as the sole control material, has limitations for the nutritional assessment, principally because of an inability to detect unintended effects. The use of low energy diets (about 3–3.5 % fat) further reduced the capacity of the study to detect unintended effects. As the diets were formulated to provide the same nutrition, the expectation was that chicken from the five experimental groups would show essentially the same performance characteristics. Results confirmed the nutritional value of soybean toasted defatted meal derived from soybean 305423, as the zootechnical performance parameters of the test group were within the 95 % tolerance intervals derived from the three commercial non-GM varieties. Laying hen feeding study and pig feeding study: as the diets were formulated to provide the same nutrition, the expectation was that hens (resp. pigs) from the four experimental groups would show essentially the same performance characteristics. Results confirmed the nutritional value of soybean toasted defatted meal and the absence of any unintended effects impacting on performance at the tested level. | 7/31/2014 | Negative opinion | No reservation | The elements provided by the applicant to complement the molecular characterisation don't allow to lift all the reservations expressed in the 2008 opinion, in particular those related to the presence of 3 unnecessary insertions. | Reservations | The compositional analyses provided don't allow to conclude that soybean 305423 is equivalent to conventional soybean varieties. Additionally, no analysis was performed on the products derived from this soybean, especially the oil. | Reservations | The two 90-day studies provided don't allow to conclude about the possible toxicological effects linked to a repeated consumption of the oil intended for human consumption and of the meal intended for use as feed. | No reservation | Reservations | The trials presented don't allow to conclude that all the grain elements of soybean 305423 are nutritionally equivalent to those of conventional soybeans. This equivalence is demonstrated for the meal but not for the hulls not for the oil. | |||||||||||||
| Soybean | 305423 | Pioneer | EFSA-GMO-NL-2007-45 (Regulation (EC) No 1829/2003) |
- | - | 1/21/2008 | Negative opinion | Reservations | Soybean 305423 contains 4 independent insertions, comprising several entire or truncated PHP19340A et PHP17752A fragments, as well as a region of the plasmid used for the genetic construction. Therefore soybean 305423 is not satisfactory on a molecular point of view and the 3 insertions unnecessary for the expression of the 2 intended traits should have been removed. | Reservations | The compositional analytical data don't allow to conclude to the substantial equivalence of soybean 305423 and its control, because in particular of heptadecanoic acid (C17 :0) and heptadecenoic acid (C17 :1) higher levels in the transgenic plant. Additionally, it would have been desirable to complement the analyses in accordance with the codex alimentarius (2007) and OECD (2002) recommendations concerning the comparative assessment of the composition of the oil (phytosterols), lecithin and proteins isolated from soybean 305423. | Reservations | No repeated-dose 90-day oral toxicity study in rodents on whole food/feed. | No reservation | None of the 27 newly created ORFS have structural identities with proteins known to have allergenic, toxic or anti-nutritional properties. A study performed with sera from patients allergic to soybean has been performed. The results show that the qualitative and quantitative profile of allergenic proteins in soybean 305423 is not different from that of the isogenic control. Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | Reservations | The diets of the poultry trial contain only 0.5% of oil. Therefore, it is not possible to conclude on the potential effects of the modification of the oil fatty acid composition and on the nutritional equivalence of soybean 305423 and its control. | ||||||||||||||||||||
| Soybean | MON87751 | Monsanto | EFSA-GMO-NL-2014-121 (Regulation (EC) No 1829/2003) |
8/2/2018 | Positive opinion | No reservation | No reservation | No reservation | No reservation | Pending question about the possible adjuvanticity of the Cry proteins with respect to the publication of Vazquez on Cry1Ac. | No reservation | 4/15/2015 | Positive opinion | No reservation | Pending question about the pests targeted by Cry1A.105 and Cry2Ab2. | No reservation | No analyses available on the products obtained from soybean MON87751. | No reservation | No insights available on a possible risk associated to the soybean oil consumption. | No reservation | Probable absence of allergenicity for the refined soybean oil. | No reservation | Not necessary due to the absence of significant discrepancies in the comparative analysis. | ||||||||||||||
| Cotton | MON88701 | Monsanto | EFSA-GMO-NL-2013-114 (Regulation (EC) No 1829/2003) |
3/30/2017 | Inconclusive opinion | No reservation | Inconclusive | Complete compositional results reported for only three sites. | Inconclusive | No 28-day toxicity study in rodents on the MON88701 DMO protein. | Inconclusive | The safety assessment of the DMO protein in cotton MON88701 could not be completed. | Inconclusive | The safety assessment of the DMO protein in cotton MON88701 could not be completed. | 10/11/2013 | Negative opinion | No reservation | No reservation | For a few compounds (carbohydrates, fibres, ash), it is not possible to conclude that cotton MON88701 is equivalent to commercial varieties, but these compounds do not present a danger to human or animal health. | Reservations | Absence of a repeated-dose 90-day oral toxicity study on whole food/feed in rodents. | No reservation | Reservations | No feeding study in target animals. | |||||||||||||
| Cotton | T304‐40 | Bayer CropScience AG | EFSA-GMO-NL-2011-97 (Regulation (EC) No 1829/2003) |
7/29/2013 | Positive opinion | No reservation | No reservation | The differences for insect damage and presence of insect larvae were expected, as cotton T304-40 expresses the insecticidal Cry1Ab protein. All the other statistically significant differences were within the natural variation of reference varieties. | No reservation | No reservation | No reservation | Having considered the design and outcome of the feeding study with chicken for fattening, the Panel notes that the value of the study is limited by (i) the high mortality observed and (ii) the low dietary inclusion level of cotton seed. | 10/11/2013 | Negative opinion | Reservations | The mortality and morbidity rates are abnormally high. The explanations given for the cause of death are admissible but the number of groups is greatly reduced, and therefore the power of the test is insufficient. | |||||||||||||||||||||
| Cotton | T304‐40 | Bayer CropScience AG | EFSA-GMO-NL-2011-97 (Regulation (EC) No 1829/2003) |
- | - | 1/23/2012 | Negative opinion | No reservation | No reservation | Some statistically significant differences are observed in the trials. All these differences correspond to small variations and the means are within the range of values of the public data of the main cotton varieties. | No reservation | No reservation | Reservations | Mortality observed in the study is abnormally high (greater than 10% regardless of the diet). | |||||||||||||||||||||||
| Maize | DAS‐40278‐9 | DOW AgroSciences LLC | EFSA-GMO-NL-2010-89 (Regulation (EC) No 1829/2003) |
12/5/2016 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 6/7/2011 | Negative opinion | No reservation | No reservation | Reservations | The lack of a 90-day sub-chronic toxicity study with whole food does not allow an assessment of the potential risk associated with the consumption of food products derived from DAS-40278-9 maize. | Given the small number of animals used for the 28-day study (5 of each sex per group), however corresponding to the OECD experimental protocol, the risk of having insufficient power for statistical tests of differences increases. No 90-day sub-chronic toxicity study in rats with food has been performed. | No reservation | No reservation | ||||||||||||||||||
| Maize | MIR162 | Syngenta | EFSA-GMO-DE-2010-82 (Regulation (EC) No 1829/2003) |
6/21/2012 | Positive opinion | No reservation | No reservation | Some statistically significant differences were observed (for ex C18:2) but they fell in the range of biblio data. | No reservation | A 90-day study is provided. | No reservation | No reservation | Poultry trial: maize MIR162 is as nutritious as the conventional counterpart. | 11/17/2010 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | |||||||||||||||||
| Soybean | BPS-CV127-9 | BASF Plant Science | EFSA-GMO-NL-2009-64 (Regulation (EC) No 1829/2003) |
1/17/2014 | Positive opinion | No reservation | No reservation | Increase of seed weight which does not affect the safety of the GM soybean. | No reservation | No reservation | No reservation | GM soybean as nutritious as non GM soybean. | 3/6/2014 | Positive opinion | No reservation | The 90-day study, performed with toasted defatted soybean meals, doesn't reveal effects of biological significance. Given the low oil content of the meals, the study can't document the risk linked to the oil, which on another note doesn't contain the newly expressed protein. This study, carried out before the Anses and EFSA recommendations (2011), doesn't include an analysis of the power of the statistical tests. | No reservation | Updated bioinformatic analyses (2013) and a study of the protein degradation in simulated digestion medium (2013) were assessed. | |||||||||||||||||||
| Soybean | BPS-CV127-9 | BASF Plant Science | EFSA-GMO-NL-2009-64 (Regulation (EC) No 1829/2003) |
- | - | 10/20/2009 | Negative opinion | No reservation | No reservation | Metabolic intermediates resulting from the AHAS activity should have been studied. | Reservations | No sub-chronic 90-day study was performed. | No reservation | No reservation | |||||||||||||||||||||||
| Soybean | DAS‐68416‐4 | Dow AgroSciences LLC | EFSA-GMO-NL-2011-91 (Regulation (EC) No 1829/2003) |
3/16/2017 | Positive opinion | No reservation | No reservation | No relevant differences except for ‘days to 50% flowering’ and an increase (up to 36%) in lectin activity . | No reservation | No reservation | No reservation | 12/6/2011 | Negative opinion | No reservation | Reservations | No equivalence of the lectin content of the seed. | Reservations | Because of the difference in the levels of lectins, the origin of the haematological changes should be documented and no consideration of the statistical power assessment of the test (may be insufficient with 12 rats per group). | No reservation | No reservation | |||||||||||||||||
| Soybean | FG72 | Bayer CropScience | EFSA-GMO-BE-2011-98 (Regulation (EC) No 1829/2003) |
7/16/2015 | Positive opinion | No reservation | No reservation | The soybean meals used should have come from soybeans treated with herbicides. | No reservation | The soybean meals used should have come from soybeans treated with herbicides. | No reservation | No reservation | Overall mortality was high (10.7 %) with no significant difference between the groups. The soybean meals used should have come from soybeans treated with herbicides. | 1/19/2012 | Positive opinion | No reservation | Bioinformatics analyses predicted the presence of 3 potential genes in the transgene insertion region. One of them, that would code for a potential cysteine protease, is in the fragment that has undergone the translocation. In this new organization, the sequence encoding the putative cysteine protease is found downstream of a potential new promoter. The applicant has not verified whether the gene is expressed in FG72 soybeans or not. | No reservation | Equivalence tests as recommended by EFSA (EFSA, 20104) are not performed. The analysis of the composition of the seed was supplemented by data on the whole plant (forage) and transformation products of the seed. No statistical analysis was performed. The analysis of the composition data does not reveal any major difference according to the genetic origin of the seeds from which the products are derived. | No reservation | The use of a small number of animals (10 rats of each sex per group) increases the risk of having insufficient power for statistical tests. The soybean meals used should have come from soybeans treated with herbicides. | No reservation | No reservation | The soybean meals used should have come from soybeans treated with herbicides. | |||||||||||||
| Soybean | MON87701 | Monsanto | EFSA-GMO-BE-2010-79 (Regulation (EC) No 1829/2003) |
7/26/2011 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 10/10/2011 | Positive opinion | No reservation | Additional elements showing no statistically significant difference between the GMO fed chickens compared to the control group. | |||||||||||||||||||||||
| Soybean | MON87701 | Monsanto | EFSA-GMO-BE-2010-79 (Regulation (EC) No 1829/2003) |
- | - | 8/24/2010 | Negative opinion | No reservation | No reservation | No reservation | No reservation | Reservations | No statistical study relating to a higher mortality of chickens fed with GMOs. | Higher mortality rate in the group of chickens fed with the GMO. No explanation from the applicant. | |||||||||||||||||||||||
| Soybean | MON87708 | Monsanto | EFSA-GMO-NL-2011-93 (Regulation (EC) No 1829/2003) |
10/3/2013 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 7/28/2011 | Negative opinion | Reservations | No explanation on the introduction of the 2 T-DNA and the elimination of the glyphosate resistance trait. | Reservations | Equivalence of the composition of MON87708 with control soybean is not supported with appropriate statistical tests. | Reservations | The 90-day test has not been conducted with 87708 treated with the herbicide Dicamba. The number of animals per sex and group is too low (n=12). | Reservations | The safety of soybean oil for human consumption is not documented. | No reservation | ||||||||||||||||
| Soybean | MON87769 | Monsanto | EFSA-GMO-UK-2009-76 (Regulation (EC) No 1829/2003) |
5/16/2014 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 5/11/2010 | Negative opinion | No reservation | No reservation | Reservations | Regarding the PjD6D and NcD15D proteins, the doses used in the acute toxicity studies are much lower than those usually used in such studies. The safety of the soybean MON87769 grain cannot be evaluated in the absence of a 90-day toxicity study performed with plant material containing the lipidic fraction. The oil from soybean MON87769 should be evaluated as a novel food, according to regulation 258/97/CE, because no other oil or food consumed to this day contain so much SDA. | The defatted fraction of the grain intended for use as feed is considered to be as safe as the meal from the control or conventional soybean varieties. | No reservation | Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | Reservations | The study, performed with a defatted fraction of the grain, doesn't allow to conclude to the nutritional equivalence of the grain or the oil from soybean MON87769. No study of the fatty acid profile changes in the products derived from animals fed soybean MON87769 is provided, whereas the applicant claims the use of this soybean to increase the omega 3 content of the animal products, which should be assessed since they are intended for human consumption. | ||||||||||||||||
| Cotton | GHB614 x LLCotton25 | Bayer CropScience | EFSA-GMO-NL-2010-77 (Regulation (EC) No 1829/2003) |
5/16/2014 | Positive opinion | No reservation | No reservation | Higher level of gossypol but no incidence on safety. | No reservation | Given that no adverse effects in the available toxicological studies were observed, and since there were no structural similarities to known toxins, the "response addition model" predicts that the newly expressed proteins in the two-event stack cotton would not give rise to safety concerns for human and animal health. | No reservation | The newly expressed proteins in cotton GHB614 x LLCotton25 have been individually assessed previously and no safety concerns were identified. There are no new data, which would lead to a revision of these conclusions. Moreover, no interactions between the newly expressed proteins are expected based on their biological properties. | No reservation | 4/4/2011 | Negative opinion | Reservations | Absence of a 90-day sub-chronic toxicity study in rats conducted by repeated administration of the seed, or its derived products (cake and oil), from the parental cotton plants or the cotton plant containing the two transformation events. | ||||||||||||||||||||
| Cotton | GHB614 x LLcotton25 x MON15985 | Bayer CropScience AG | EFSA-GMO-NL-2011-94 (Regulation (EC) No 1829/2003) |
4/20/2018 | Positive opinion | No reservation | No reservation | The differences identified in the agronomic and phenotypic characteristics between cotton GHB614 x LLCotton25 x MON15985 and the non-GM comparator do not require further assessment regarding food and feed safety. The significant compositional differences between cotton GHB614 x LLCotton25 x MON15985 and the non-GM comparator (10 and 14 endpoints for the untreated and treated plants, respectively) don't need further food/feed safety assessment, except for gossypol, dihydrosterculic acid and a-tocopherol, which are assessed in Section 3.5. | No reservation | No reservation | No reservation | The GMO Panel is able to draw only limited conclusions from this study, because of the high mortality and the number of clinical signs observed. However, the measured performance endpoints were similar in groups fed balanced diets containing 10% of GM and non-GM cottonseed meals. | 4/1/2016 | Negative opinion | Reservations | Presence of the aad antibiotic resistance gene for cotton MON15985. | Reservations | 90-day sub-chronic toxicity study performed with diets containing no oil nor seeds for cotton GHB614 and no 90-day sub-chronic toxicity study for cotton LLcotton25. | |||||||||||||||||||
| Soybean | MON87701 x MON89788 | Monsanto | EFSA-GMO-NL-2009-73 (Regulation (EC) No 1829/2003) |
2/15/2012 | Positive opinion | No reservation | No reservation | No biologically relevant differences in the composition or agronomic and phenotypic characteristics of soybean MON87701 x MON89788 as compared with the comparator soybean A5547. The composition of soybean MON87701 x MON89788 falls within the range observed in non-GM soybean varieties. No interaction that cause compositional, agronomic or phenotypic changes that would raise safety concerns. | No reservation | No additional animal safety studies are required. | No reservation | No reservation | No relevant difference in broiler performance. Soybean MON87701 x MON89788 is as nutritious as conventional soybeans. | 4/4/2012 | Positive opinion | No reservation | A 90-day study performed on soybean MON88701 was provided in an other application. | ||||||||||||||||||||
| Soybean | MON87701 x MON89788 | Monsanto | EFSA-GMO-NL-2009-73 (Regulation (EC) No 1829/2003) |
- | - | 3/3/2010 | Negative opinion | No reservation | Soybean MON87701 has not been assessed previously. | No reservation | Reservations | Absence of 90-day study. | No 90-day study for MON87701 or MON87701 x MON89788. | No reservation | No reservation for MON88701, no reservation for MON89788 but no specific analysis for the stacked soybean. | No reservation | |||||||||||||||||||||
| Soybean | MON87769 x MON89788 | Monsanto | EFSA-GMO-NL-2010-85 (Regulation (EC) No 1829/2003) |
10/8/2015 | Inconclusive opinion | No reservation | No reservation | No reservation | No reservation | Inconclusive | Lack of data on dietary exposure to refined bleached deodorised oil from soybean MON87769 x MON89788. | The applicant was asked to provide a dietary exposure assessment based on the compositional analysis of the refined bleached deodorised oil from soybean MON87769 x MON89788, taking into account different exposure scenarios, covering low and high consumer groups. However, the applicant did not provide this data. | 7/28/2014 | Negative opinion | No reservation | Reservations | The phenotypic and agronomic characterisation of soybean MON87769 x MON89788 was carried out using crops not treated with glyphosate. The compositional analysis was only performed with soybean treated with glyphosate. The characteristics of the experimental designs do not comply with the EFSA (2006) recommendations in force for this dossier. | No reservation | No reservation | Reservations | A study comparing the effect of the origin of the oils on chicken growth and carcass yield is missing. | Since soybean MON87769 x MON89788 has been modified for its fatty acid composition, a study comparing the effect of the origin of the oils on chicken growth and carcass yield with an analysis of the fatty acid composition of the meat would have been welcome, as soybean oil is also used in animal feed. | |||||||||||||||
| Soybean | MON87705 x MON89788 | Monsanto | EFSA-GMO-NL-2011-100 (Regulation (EC) No 1829/2003) |
7/16/2015 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 1/18/2013 | Negative opinion | No reservation | No reservation | Reservations | Absence of 90-day study. | No reservation | No reservation | |||||||||||||||||||
| Soybean | MON87708 x MON89788 | Monsanto | EFSA-GMO-NL-2012-108 (Regulation (EC) No 1829/2003) |
6/18/2015 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 12/30/2013 | Positive opinion | No reservation | No reservation | No analysis has been performed on products derived from soybean MON87708 x MON89788. | No reservation | The absence of interaction between the proteins expressed in soybean MON87708 x MON89788 is not sufficiently documented. A feeding study would allow to lift the reservations that could be expressed thereon. | No reservation | No reservation | ||||||||||||||||||
| Soybean | 305423 x 40-3-2 | Pioneer | EFSA-GMO-NL-2007-47 (Regulation (EC) No 1829/2003) |
8/18/2016 | Positive opinion | No reservation | No indication of interaction that may affect the integrity of the events and the levels of the newly expressed proteins in this stack. Based on the known biological function of the newly expressed proteins, no foreseen interactions at the biological level are expected. | No reservation | Soybean 305423 x 40-3-2 differs from the non-GM comparator Jack and the negative segregant, and is not equivalent to the non-GM soybean reference varieties, in having an altered fatty acid profile (the intended trait). The altered fatty acid profile is assessed in Section 3.5.5. No further assessment for food and feed safety was needed for the other differences, or any other lack of equivalence. The comparison with the two parental lines did not reveal any potential interaction that could be of concern for food and feed safety. | No reservation | Limited information is provided on the 90-day study design, material and methods and results, as well as on its GLP compliance status. The number of experimental units per group (two per sex) is low and an appropriate statistical analysis of the data to draw relevant conclusions is not possible. But a subchronic feeding study in rodents on soybean 305423 x 40-3-2 is not needed on the basis of the molecular characterisation and comparative assessment. | No reservation | No reservation | The feeding study in chickens for fattening performed with material derived from a negative segregant as the sole control material has limitations. The applicant provided another study with the same design, in which the non-GM comparator Jack was compared to another GM soybean and to the same three commercial varieties used in the first study. The GMO Panel accepts that the two studies taken in conjunction provide evidence that the defatted toasted GM soybean meal 305423 x 40-3-2 is as nutritious as non-GM soybean varieties. | 5/9/2008 | Negative opinion | No reservation | Soybean 305423 contains 4 independent insertions, 3 of which are truncated and useless for the expression of the 2 intended characters. It would be desirable to obtain an explanation for the simultaneous presence of these four insertions in the double mutant as in soybean 305423. | Reservations | The composition data do not allow to conclude to the substantial equivalence between soybean 305423 x 40-3-2 and its control, in particular because of the heptadecanoic and heptadecenoic acids concentrations which are higher in the GM plant. Additionally, it would have been desirable to supplement the data with a comparative analysis of oil composition, lecithin and isolated soybean proteins. | Reservations | Absence of 90-day study. | No reservation | Reservations | The composition of the diets used in the feeding study in broiler chickens, which contain only 0.5% of soybean oil, does not allow to conclude on the possible effects of the modification of the fatty acid composition of the oil and on the nutritional equivalence of soybean 305423 x 40-3-2 and its control. | ||||||||||||
| Soybean | FG72 x A5547-127 | Bayer CropScience LP and M.S. Technologies LLC | EFSA-GMO-NL-2013-120 (Regulation (EC) No 1829/2003) |
4/6/2017 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 11/9/2015 | Negative opinion | Reservations | The molecular characterisation must be completed by sequencing the insert and its flanking regions and analysing the expression of the putative cysteine protease (situated downstream of a putative promoter after the translocation). | No sequencing has been done on soybean FG72 x A5547-127, which is not in conformity with Regulation (UE) n° 503/2013. | No reservation | No analysis has been carried out on soybean forage nor on products derived from FG72 x A5547-127. | Reservations | In 2008, Afssa already stated that since no 90-day toxicity assay on rats fed with (product derived from) soybean A5547-127, treated or not with glufosinate-ammonium, had been provided, it could not be concluded that transformed products from soybean A5547-127 are safe. The situation is not changed and reservations on soybean A5547-127 are maintained. | The analysis of the potential interactions between the expression products of the genes introduced in soybean FG72 x A5547-127 was performed according to EFSA's recommendations. The safety assessment of the 2mEPSPS, HPPD W336 et PAT proteins doesn't reveal elements allowing to conclude that these proteins have a toxicological effect on human and animal health. | No reservation | Expression of proteins 2mEPSPS, HPPD W336 and PAT in this soybean does not seem to modify seed allergenicity compared with natural allergenicity of soybean seeds. On the basis of these elements, allergenicity potential of products derived from soybean FG72 x A5547-127 seems quite low. | No reservation | No nutritional assessment has been done, because the applicant considers that composition equivalence has been demonstrated between soybean FG72 x A5547-127 and conventional soybean. | |||||||||||||
| Soybean | MON87705 x MON87708 x MON89788 | Monsanto Company | EFSA-GMO-NL-2015-126 (Regulation (EC) No 1829/2003) |
5/18/2020 | Inconclusive opinion | No reservation | No indication of interaction that may affect the integrity of the events or the levels of the newly expressed proteins or dsRNAs in this three-event stack soybean. Based on the known biological function of the newly expressed proteins and dsRNA, no foreseen interactions at the biological level are expected. | No reservation | The changes in the fatty acid profile in seeds are consistent with the intended trait and assessed in Section 3.6.6. Compositional differences between soybean MON87705 x MON87708 x MON89788 and the non-GM comparator were also identified for Gly m 3 and total fat and are further assessed in Sections 3.6.4 and 3.6.6, respectively. | Inconclusive | The applicant did not provide a 90-day study on MON87705 soybean in line with the applicable legal requirements. | No reservation | Allergen Gly m 3 levels in soybean MON87705 x MON87708 x MON89788 (treated) were significantly different from those of the non-GM comparator and fell under equivalence category III. The difference reported for this allergen consists in a decrease and no relevant differences in the content of other allergens were observed. Thus, no changes in the levels of endogenous allergens raising concern are identified by the GMO Panel. | No reservation | The consumption of soybean MON87705 x MON87708 x MON89788 does not represent any nutritional concern, in the context of the scope of this application. | 5/11/2016 | Negative opinion | Reservations | In line with Implementing Regulation No. 503/20132, the applicant must provide Southern blot analyses carried out on soybean MON87705 x MON87708 x MON89788. Additionally, the risk assessment of off-target effects linked to the use of RNA interference should not only be performed on insects but also on the genetically modified plant (GMP) and humans and animals that will consume this GMP. | No reservation | The compositional analysis performed on raw grains and forage, as well as the phenotypic and agronomic characterisation of soybean MON87705 x MON87708 x MON89788, treated or not with glyphosate and dicamba, show that this soybean is equivalent to conventional soybean varieties. The only exceptions are the expected change in the fatty acid profile of the grains and the unexpected change in the levels of 3 minor fatty acids that each represent less than 0.4% of total fatty acids. No analysis of products derived from soybean MON87705 x MON87708 x MON89788, notably lipidic products such as oil. | Reservations | The applicant must provide the acute toxicity studies in mice carried out in 2015 on the CP4 EPSPS et DMO proteins. 90-day toxicity studies have been provided for each of the parental soybeans. Nevertheless, the soybean MON87705 study was performed on defatted meal, which doesn't document the security of the oil, the composition of which is intentionally modified. A 90-day study performed on soybean MON87705 x MON87708 x MON89788 derived products containing the lipidic fraction is therefore necessary. The soybean will have to be treated with glyphosate and dicamba. Lastly, the applicant doesn't analyse the potential interactions between the MON87705, MON87708 and MON89788 events in the stack. | No reservation | Reservations | A study comparing the effect of the origin of the oils on chicken growth and carcass yield together with an analysis of the meat fatty acid composition is necessary, since the oil is also used for animal feeding. | |||||||||||
| Maize | Bt11 x MIR604 | Syngenta Seeds | EFSA-GMO-UK-2007-50 (Regulation (EC) No 1829/2003) |
5/18/2010 | Positive opinion | No reservation | No reservation | The comparative assessment of maize Bt11 x MIR604 was based on the compositional analysis of forage and grain derived from maize Bt11 x MIR604 x GA21 which is in accordance with the EFSA GMO Panel’s guidance document on GM plants containing stacked transformation events (EFSA, 2007a). The EFSA GMO Panel accepted the use of maize Bt11 x MIR604 x GA21 for the comparative compositional analysis and concludes that forage and grain from the maize Bt11 x MIR604, assessed in this application, are likely to be compositionally equivalent to those of its conventional counterpart except for the presence of the newly expressed proteins. | No reservation | Based on the known function and mode of action of the newly expressed proteins Cry1Ab, PAT, mCry3A and PMI, the EFSA GMO Panel considers the occurrence of interactions between these proteins unlikely. | No reservation | No reservation | This study was not considered by the EFSA GMO Panel because of relevant deviations from Good Agricultural Practice (e.g., ILSI 2003, 2007), in particular very high temperatures and high animal losses in the finishing period as well as large differences in crude protein content of grower diets. | 6/10/2008 | Positive opinion | No reservation | No reservation | The comparative assessment of maize Bt11 x MIR604 was based on the compositional analysis of forage and grain derived from maize Bt11 x MIR604 x GA21 which is in accordance with the EFSA GMO Panel’s guidance document on GM plants containing stacked transformation events (EFSA, 2007a). Afssa accepted the use of maize Bt11 x MIR604 x GA21 for the comparative compositional analysis and concludes that forage and grain from the maize Bt11 x MIR604, assessed in this application, are likely to be compositionally equivalent to those of its conventional counterpart except for the presence of the newly expressed proteins. | No reservation | No reservation | No reservation | Study considered admissible by Afssa. | |||||||||||||||
| Maize | MZIR098 | Syngenta Crop Protection N.V./S.A. | EFSA-GMO-DE-2017-142 (Regulation (EC) No 1829/2003) |
6/26/2020 | Positive opinion | No reservation | Updated bioinformatic analyses of the newly created ORFs within the insert or spanning the junctions between the insert and genomic DNA revealed a single ORF which exceeded the allergenicity assessment threshold of 35% identity using an 80 amino acid sliding window approach. For the assessment of this ORF, the GMO Panel followed a weight of evidence approach and is of the opinion that this ORF does not raise concerns that need additional food/feed safety considerations. |
No reservation | None of the identified differences in the compositional characteristics tested between maize MZIR098 and its conventional counterpart needs further assessment, with the exception of NDF in grains. The NDF difference in grains was further assessed for safety and nutritional relevance and raises no concerns. | No reservation | The GMO Panel notes that the applicant only tested 41.5% dose level with the full set of OECD parameters; this incorporation rate of maize is in line with commercially available rodent diets. It has been recently reported that a diet incorporating 50% maize may be tolerated without inducing nutritional imbalances in rats after 90-day administration (Steinberg et al., 2019), but the GMO Panel considers that further scientific confirmation is needed before this 50% maize incorporation rate is applicable in future studies. | No reservation | No reservation | 10/7/2020 | Negative opinion | No reservation | Reservations | The reservation regarding the mention of a treatment with glyphosate is maintained. The standard deviations or the range of values of the compositional data for the commercial reference varieties would be useful. | Reservations | A second 90-day toxicity study has been provided but limitations in the design (10 rats/group/sex), incomplete historical data and no power analysis. | |||||||||||||||||
| Maize | MZIR098 | Syngenta Crop Protection N.V./S.A. | EFSA-GMO-DE-2017-142 (Regulation (EC) No 1829/2003) |
- | - | 5/23/2018 | Negative opinion | No reservation | Reservations | Imprecisions regarding the control used in the trials and the reasons why 2 sites were excluded from the analysis. Mention of an herbicide treatment with glyphosate, to which maize MZIR098 is not tolerant, which casts doubt on the fact that the data provided in the application are related to this maize and not to another GMO. Plants treated with glufosinate-ammonium in this trial, so the applicant should have mentioned tolerance to this herbicide among the claimed characters. | Reservations | Information is lacking regarding the 90-day toxicity study, in particular the power analysis. | Necessary to confirm that the GM maize was treated with glufosinate-ammonium. Statistical model used by the applicant considered complex. Results tables don't explicitly mention the parameters for which significant differences are observed. Number of animals (10 rats/group/sex) not in line with the recommendations of Anses (2011b) and the Scientific Committee of EFSA (2011). No power analysis, whereas it is mandatory according to Regulation (UE) n° 503/2013. |
Reservations | Information is lacking to explain why the allergenicity of maize MZIR098 can be considered comparable to that of conventional corn. | Reservations | No feeding study. | No nutritional assessment has been done, because the applicant considers that composition equivalence has been demonstrated between maize MZIR098 and conventional maize. | |||||||||||||||||||
| Rapeseed | MS8 x RF3 x GT73 and subcombinations, which have not been authorised previously (i.e. MS8 x GT73 and RF3 x GT73) independently of their origin | Bayer CropScience and Monsanto | EFSA-GMO-NL-2009-75 (Regulation (EC) No 1829/2003) |
7/30/2020 | Positive opinion | No reservation | A 28-day toxicity study in mice has been provided and assessed. | 7/4/2016 | Negative opinion | No reservation | The levels of the PAT, CP4 EPSPS and GOXv247 proteins have been measured in different tissues collected at different development stages on plants cultivated at 3 sites in Canada in 2011, that were treated with glyphosate and glufosinate-ammonium. | Reservations | No equivalence with commercial varieties | Reservations | No 90-day toxicity study. | No reservation | Reservations | A feeding study on a product derived from stacked rapeseed MS8 x RF3 x GT73 is needed. | |||||||||||||||||||
| Rapeseed | MS8 x RF3 x GT73 and subcombinations, which have not been authorised previously (i.e. MS8 x GT73 and RF3 x GT73) independently of their origin | Bayer CropScience and Monsanto | EFSA-GMO-NL-2009-75 (Regulation (EC) No 1829/2003) |
5/20/2016 | Inconclusive opinion | No reservation | Protein expression analyses showed some difference between the levels in the parental lines and the stack which are not unexpected. Based on known biological function of the newly expressed proteins, functional interaction between the Barnase and Barstar proteins are expected. These proteins are expressed in plant tissues that are not present in food, or feed derived from the three-event stack oilseed rape. No functional interaction is expected for the other newly expressed proteins. | No reservation | Inconclusive | Essential data needed for the safety assessment of the GOXv247 protein were not provided by the applicant. Taking into account all available information on the safety of GOXv247 protein, a weight-of-evidence approach could not be followed to sufficiently reduce current uncertainties mainly due to the lack of a 28-day study. | No reservation | No reservation | 5/2/2013 | Negative opinion | Reservations | An analysis of the expression of the transgenes in samples of MS8 x RF3 x GT73 and parental oilseed rape field-grown simultaneously with and without treatment with herbicides (glyphosate, glufosinate), at 3 sites or 3 seasons, is missing. | Reservations | The list of compounds is incomplete as compared to the OECD document. The statistical analysis is not in accordance with EFSA recommendations. | Reservations | No 90-day toxicity study, aimed at eliminating the risk of potential interactions between the newly expressed proteins. | No reservation | Reservations | A feeding study on a product derived from stacked rapeseed MS8 x RF3 x GT73 is needed to supplement the comparative composition analysis data. | ||||||||||||||
| Maize | Subcombination Bt11 x MIR162 related to the application EFSA-GMO-DE-2009-66 | Syngenta | EFSA-GMO-DE-2009-66 (Regulation (EC) No 1829/2003) |
3/24/2017 | Positive opinion | No reservation | The molecular characterisation was reduced to the analysis of the levels of the newly expressed proteins Cry1Ab, PAT, Vip3Aa20 and PMI and to the comparison of these levels between the two-event stack Bt11 x MIR162 and the corresponding single events Bt11 and MIR162. Based on the data provided by the applicant, EFSA concludes that there is no indication of an interaction between the events that would affect the levels of the newly expressed proteins in this two-event stack maize Bt11 x MIR162. | - | - | ||||||||||||||||||||||||||||
| Maize | The ten subcombinations of Bt11 x MIR162 x MIR604 x GA21 | Syngenta | EFSA-GMO-DE-2009-66 (Regulation (EC) No 1829/2003) |
- | - | 2/18/2016 | Positive opinion | No reservation | Conclusion based on previous assessments done on Bt11 x MIR162 x MIR604 x GA21, its parents and several subcombinations. In case varieties corresponding to the different subcombinations are produced by conventional breeding, this conclusion only applies if the transformation events used in the crosses are identical (sequences of the inserts and location of these sequences in the plant) to those that were assessed and are authorised. | No reservation | Conclusion based on previous assessments done on Bt11 x MIR162 x MIR604 x GA21, its parents and several subcombinations. | No reservation | Conclusion based on previous assessments done on Bt11 x MIR162 x MIR604 x GA21, its parents and several subcombinations. | No reservation | Conclusion based on previous assessments done on Bt11 x MIR162 x MIR604 x GA21, its parents and several subcombinations. | ||||||||||||||||||||||
| Maize | Bt11 x MIR162 x MIR604 x GA21 and subcombinations independently of their origin | Syngenta | EFSA-GMO-DE-2009-66 (Regulation (EC) No 1829/2003) |
12/7/2015 | Inconclusive opinion | Inconclusive | No data provided for some subcombinations (Bt11 x MIR162 x MIR604, MIR162 x MIR604 x GA21, Bt11 x MIR162, MIR162 x MIR604, MIR162 x GA21). No reservation for the other combinations. | The EFSA GMO Panel recommends that the applicant collate relevant information, if these subcombinations were to be created via targeted breeding approaches and commercialised in the future. In this case, this information should focus on expression levels of the newly expressed proteins. | No reservation | Reported for Bt11 × MIR162 × MIR604 × GA21 and several subcombinations. | No reservation | Reported for Bt11 × MIR162 × MIR604 × GA21. | No reservation | Reported for Bt11 × MIR162 × MIR604 × GA21. | No reservation | Reported for Bt11 × MIR162 × MIR604 × GA21. | 10/22/2009 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | ||||||||||||||
| Maize | MON87411 | Monsanto | EFSA-GMO-NL-2015-124 (Regulation (EC) No 1829/2003) |
- | - | 1/23/2019 | Reserved opinion | No reservation | New data provided that allow to lift the reservations expressed in the previous opinion. | No reservation | Reservations | Issue in the statistical analysis. The assumptions made by the applicant for its power statistical analysis are not fully justified. | No reservation | No reservation | A broiler chicken feeding study and a catfish feeding study were provided and assessed. | ||||||||||||||||||||||
| Maize | MON87411 | Monsanto | EFSA-GMO-NL-2015-124 (Regulation (EC) No 1829/2003) |
6/28/2018 | Positive opinion | No reservation | No reservation | A non-equivalence between the GM maize and non-GM reference varieties for palmitic acid was reported. The same non-equivalence was also reported for the non-GM counterpart of the GM maize. | No reservation | No reservation | No reservation | 11/10/2015 | Negative opinion | Reservations | Information is lacking concerning the comparator used in the analysis of the levels of the Cry3Bb1 and CP4 EPSPS proteins and of the RNA directed toward the DvSnf7 gene in grains and forage. The assessment of the off-target effects was not performed specifically with the RNA sequence directed toward the DvSnf7 gene. | No reservation | High concentration of palmitic acid in the grains mentioned, but not considered as problematic. | Reservations | Insufficient information concerning the potential toxicity of the RNA directed toward the gene DvSnf7. | No reservation | No feeding study. | ||||||||||||||||
| Maize | All sub-combinations of MON89034 x 1507 x NK603 independently of their origin | Dow AgroSciences and Monsanto | EFSA-GMO-NL-2009-65 (Regulation (EC) No 1829/2003) |
9/28/2011 | Positive opinion | No reservation | Assessment based on previous EFSA opinions. | No reservation | Assessment based on previous EFSA opinions. | No reservation | Assessment based on previous EFSA opinions. | No reservation | Assessment based on previous EFSA opinions. | No reservation | Assessment based on previous EFSA opinions. | - | - | ||||||||||||||||||||
| Maize | MON89034 x 1507 x NK603 and all sub-combinations of the individual events as present in its segregating progeny | Dow AgroSciences and Monsanto | EFSA-GMO-NL-2009-65 (Regulation (EC) No 1829/2003) |
10/15/2010 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 10/26/2009 | Negative opinion | Reservations | No new 90-day toxicity study in rats was reported for MON89034 x 1507 x NK603 and a former 90-day toxicity study in rats, conducted on MON89034, previously received a negative opinion from Afssa. | |||||||||||||||||||||||
| Soybean | A5547-127 | Bayer CropScience | EFSA-GMO-NL-2008-52 (Regulation (EC) No 1829/2003) |
- | - | 3/16/2020 | Negative opinion | Reservations | Suspected hepatotoxicity according to the results of the 90-day toxicity study in rats. Statistical power analysis not fully satisfactory. | ||||||||||||||||||||||||||||
| Soybean | A5547-127 | Bayer CropScience | EFSA-GMO-NL-2008-52 (Regulation (EC) No 1829/2003) |
- | - | 7/7/2017 | Negative opinion | No reservation | Reservations | Issues in the design and in the statistical analysis of the 90-day toxicity study in rats provided by the applicant: sample size too small, dose too low, statistical analysis not fully satisfactory, no power analysis and concerns about the application or not of herbicide. | |||||||||||||||||||||||||||
| Soybean | A5547-127 | Bayer CropScience | EFSA-GMO-NL-2008-52 (Regulation (EC) No 1829/2003) |
5/10/2011 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 10/15/2008 | Negative opinion | No reservation | No reservation | Reservations | No 90-day toxicity study in rats provided. | No reservation | No reservation | |||||||||||||||||||
| Maize | Bt11 x MIR604 x GA21 | Syngenta Seeds | EFSA-GMO-UK-2008-56 (Regulation (EC) No 1829/2003) |
5/18/2010 | Positive opinion | No reservation | New sequences were obtained in 2015 for MIR604 and GA21: differences about some bases were observed in both but these differences were also observed in the original plant material. | No reservation | No reservation | No reservation | No reservation | 11/14/2008 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | |||||||||||||||||||
| Maize | MON87427 x MON89034 x NK603 and subcombinations independently of their origin | Monsanto Company | EFSA-GMO-BE-2013-117 (Regulation (EC) No 1829/2003) |
8/1/2017 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 9/1/2015 | Negative opinion | No reservation | No reservation | Reservations | The argument concerning the potential interactions between the products of the expression of the genes introduced into the plant is superficial, not allowing to document possible interactions. The reservations previously expressed for parent MON89034 due to the abnormally high frequency of bladder stones in the 90-day sub-chronic toxicity study made with this corn are maintained. | No reservation | Reservations | Nutritional assessment of this corn was not carried out. Such a study could have helped to document the questions about potential interactions. | ||||||||||||||||||
| Rapeseed | GT73 | Monsanto | EFSA-GMO-NL-2010-87 (Regulation (EC) No 1829/2003) |
2/12/2013 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 2/13/2012 | Positive opinion | No reservation | Without calling into question the conclusions of the molecular characterization, the experts consider that the applicant should have taken advantage of updating this dossier to present longer flanking sequences (around 1000 bp on each side) in order to better define the genomic region before insertion. | No reservation | No reservation | No reservation | No reservation | |||||||||||||||||||
| Soybean | DAS-81419-2 | Dow AgroSciences | EFSA-GMO-NL-2013-116 (Regulation (EC) No 1829/2003) |
12/5/2016 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 7/27/2018 | Negative opinion | Reservations | A 90-day toxicity study was provided. Several aspects of this study are problematic: there is a doubt about the dose given to one group of rats, the applicant does not specify whether the GM soybean has been treated with the herbicide to which it is tolerant, the raw data under electronic format and calculation programs are not provided, the number of animals does not correspond to the recommendations of Anses (2011) and EFSA Scientific Committee (2011), and the applicant does not provide a power analysis. | |||||||||||||||||||||||
| Soybean | DAS-81419-2 | Dow AgroSciences | EFSA-GMO-NL-2013-116 (Regulation (EC) No 1829/2003) |
- | - | 5/23/2014 | Negative opinion | No reservation | Reservations | The compositional equivalence of genetically modified soybean DAS-81419-2 with conventional varieties is not demonstrated. Additionally, the comparative evaluation was carried out on plants cultivated without any herbicide treatment with glufosinate-ammonium, whereas soybean DAS-81419-2 contains the pat gene. | It is not possible to compare the values measured on soybean DAS-81419-2 with the ranges of values measured on the commercial varieties for the parameters the results of which are both different and non equivalent, because the raw, non transformed data, were not provided. | Reservations | No 90-day toxicity study in rats provided. | No reservation | Reservations | No feeding study. | No nutritional assessment has been done, because the applicant considers that composition equivalence has been demonstrated between soybean DAS-81419-2 and conventional soybean. | ||||||||||||||||||||
| Maize | MON87427 x MON89034 x MIR162 x NK603 and subcombinations | Monsanto | EFSA-GMO-NL-2016-131 (Regulation (EC) No 1829/2003) |
7/8/2019 | Positive opinion | No reservation | No reservation | None of the differences identified in forage and grain composition between the four-event stack maize and the non-GM comparator needs further assessment for food/feed safety. The GMO Panel has previously assessed four subcombinations and considers that its previous conclusions on these subcombinations remain valid. For the remaining six subcombinations, no experimental data have been provided. For these subcombinations, the GMO Panel assessed the possibility of interactions between the events and concluded that these combinations would not raise safety concerns. | No reservation | On the basis of the known biological function of the individual newly expressed proteins, there is currently no expectation for possible interactions relevant to the food and feed safety of the four-event stack maize. There are no safety concerns to human and animal health related to the newly expressed proteins Cry1A.105, Cry2Ab, PMI, Vip3Aa20, CP4 EPSPS and its variant CP4 EPSPS L214P in the four-event stack maize. The GMO Panel has previously assessed four subcombinations and considers that its previous conclusions on these subcombinations remain valid. For the remaining six subcombinations, no experimental data have been provided. For these subcombinations, the GMO Panel assessed the possibility of interactions between the events and concluded that these combinations would not raise safety concerns. | No reservation | No reasons for concerns regarding the simultaneous presence of the newly expressed proteins in this four-event stack maize affecting their allergenicity. The GMO Panel identified no indications of a potentially increased allergenicity of food and feed derived from the four-event stack maize with respect to that derived from the non-GM comparator. The GMO Panel has previously assessed four subcombinations and considers that its previous conclusions on these subcombinations remain valid. For the remaining six subcombinations, no experimental data have been provided. For these subcombinations, the GMO Panel assessed the possibility of interactions between the events and concluded that these combinations would not raise safety concerns. | No reservation | Comparison of the composition of maize MON87427 x MON89034 x MIR162 x NK603 with the non-GM comparator and non-GM reference varieties did not identify differences that would require further safety assessment. From these data, the GMO Panel concludes that the nutritional impact of maize MON87427 x MON89034 x MIR162 x NK603-derived food and feed is the same as that expected from the non-GM comparator and non-GM reference varieties. The GMO Panel has previously assessed four subcombinations and considers that its previous conclusions on these subcombinations remain valid. For the remaining six subcombinations, no experimental data have been provided. For these subcombinations, the GMO Panel assessed the possibility of interactions between the events and concluded that these combinations would not raise safety concerns. | 8/8/2016 | Negative opinion | Reservations | The application doesn't contain elements that would lift he reservations related to soybean MON89034. Therefore, Anses gives a negative opinion for the four-event stack maize and all of the ten subcombinations. | |||||||||||||||||||
| Rapeseed | MON88302 x MS8 x RF3 and subcombinations independently of their origin | Monsanto Company and Bayer CropScience | EFSA-GMO-NL-2013-119 (Regulation (EC) No 1829/2003) |
4/10/2017 | Positive opinion | No reservation | No reservation | No reservation | The three-event stack and the subcombinations are considered to be as safe as the single events, the non-GM counterparts and the rapeseed varieties tested. | No reservation | No concern regarding the allergenicity of the single events and the three-event stack rapeseed. | No reservation | The three-event stack and the subcombinations are considered to be as nutritious as the single events, the non-GM counterparts and the rapeseed varieties tested. | 4/22/2015 | Negative opinion | No reservation | No reservation | No analysis was performed on products derived from rapeseed MON88302 x Ms8 x Rf3, notably oil and meal. | Reservations | No 90-day toxicity study in rats for the parental rapeseed MON88302, nor for rapeseed MON88302 x Ms8 x Rf3. | No reservation | No reservation | Not necessary since the comparative analysis demonstrated that the three-event stack MON88302 x MS8 x RF3 displays a composition equivalent to that of non-GM rapeseed varieties. | ||||||||||||||
| Rapeseed | MON88302 x MS8 x RF3 and subcombinations independently of their origin | Monsanto Company and Bayer CropScience | EFSA-GMO-NL-2013-119 (Regulation (EC) No 1829/2003) |
- | - | 7/29/2014 | Negative opinion | Reservations | No 90-day toxicity study in rats for the parental rapeseed MON88302, nor for rapeseed MON88302 x Ms8 x Rf3. | ||||||||||||||||||||||||||||
| Soybean | MON87705 | Monsanto | EFSA-GMO-NL-2010-78 (Regulation (EC) No 1829/2003) |
12/17/2013 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | Having assessed total replacement, the most conservative scenario arising from both domestic and commercial use of the oil, the EFSA GMO Panel concluded that the use of soybean MON80775 oil does not impact on human health and nutrition. | - | - | ||||||||||||||||||||||||
| Soybean | MON87705 | Monsanto | EFSA-GMO-NL-2010-78 (Regulation (EC) No 1829/2003) |
10/30/2012 | Inconclusive opinion | No reservation | No reservation | No reservation | No reservation | Inconclusive | The applicant did not provide data which would allow a nutritional assessment of soybean MON87705 oil when used for commercial frying. Therefore, the nutritional assessment of soybean MON87705 oil performed by the GMO Panel in this Opinion excludes commercial frying. | A feeding trial on broiler chickens indicates that MON87705 is as nutritious as the non-GM soybean counterpart and non-GM soybean varieties tested. | 11/16/2010 | Reserved opinion | No reservation | No reservation | Reservations | Due to the lack of results dealing with the soybean oil, results from the sub-chronic 90-day toxicological study remains incomplete and does not allow a complete assessment of the MON87705 toxic potential. | No reservation | No reservation | A feeding trial on broiler chickens indicates that MON87705 is not nutritionally different from the non-GM soybean comparator. | ||||||||||||||||
| Soybean | SYHT0H2 | Syngenta | EFSA-GMO-DE-2012-111 (Regulation (EC) No 1829/2003) |
1/20/2020 | Positive opinion | No reservation | Bioinformatic analyses indicate a ~ 30% sequence identity of AvHPPD-03 to some proteins of bacterial origin annotated as haemolysins. The relevance for the safety assessment of this finding is further discussed in Section 3.3.3.1. The GMO Panel concludes that there are no indications of toxicological concerns for the AvHPPD-03 protein expressed in soybean SYHT0H2. | No reservation | None of the compositional differences between soybean SYHT0H2 and the conventional counterpart needs further assessment for food/feed safety except for seed levels of a-tocopherol and g-tocopherol, which are discussed in Section 3.3. Considering the biological relevance of these compounds the GMO Panel considers that no further toxicological assessment is needed. | No reservation | No reservation | No reservation | Soybean SYHT0H2 is considered as nutritionally equivalent to the conventional counterpart and the non-GM soybean varieties tested. | 4/9/2013 | Negative opinion | No reservation | No reservation | For most of the analysed compounds, the composition of forage and grains of soybean SYHT0H2 is equivalent to that of the control and the tested commercial varieties. Significant differences are observed for some tocopherols (a, g and d). However, considering the low level of discrepancies, no safety concern is predicted for these tocopherol changes. | Reservations | No 90-day toxicity study in rats provided. | No reservation | Reservations | A feeding trial on target animals would have allowed to complement the comparative analysis data and to reduce the risk assessment uncertainty. | ||||||||||||||
| Maize | MIR604 x GA21 | Syngenta Seeds | EFSA-GMO-UK-2007-48 (Regulation (EC) No 1829/2003) |
5/18/2010 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 7/8/2008 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | ||||||||||||||||||||
| Maize (pollen) |
MON810 | Monsanto | EFSA-GMO-NL-2012-107 (Regulation (EC) No 1829/2003) |
12/18/2012 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 7/25/2012 | Positive opinion | No reservation | No reservation | |||||||||||||||||||||||
| Maize | MON87427 | Monsanto | EFSA-GMO-BE-2012-110 (Regulation (EC) No 1829/2003) |
6/19/2015 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 9/1/2015 | Positive opinion | No reservation | No reservation | No reservation | A 90-day study was provided and assessed. | No reservation | No reservation | |||||||||||||||||||
| Maize | MON87427 | Monsanto | EFSA-GMO-BE-2012-110 (Regulation (EC) No 1829/2003) |
- | - | 4/2/2013 | Negative opinion | No reservation | No reservation | Reservations | Absence of a repeated-dose 90-day oral toxicity study on whole food/feed in rodents. | No reservation | Reservations | A feeding trial on target animals would have allowed to complement the data of the comparative analysis of the composition and to reduce the risk assessment uncertainty. | |||||||||||||||||||||||
| Maize | MZHG0JG | Syngenta | EFSA-GMO-DE-2016-133 (Regulation (EC) No 1829/2003) |
11/14/2018 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 3/31/2017 | Negative opinion | No reservation | The absence of sequences from the helper plasmid pSB1 in the plant genome should be verified, even if the presence of such sequences in the plant is very unlikely. | No reservation | Reservations | The number of animals (10 rats / group / sex) does not correspond to the recommendations of Anses (2011) and of the EFSA Scientific Committee (2011), and the applicant does not provide a power analysis, while this is a requirement of the Implementing Regulation (EU) n ° 503/2013. Under these conditions, it is not possible to conclude on this 90-day sub-chronic toxicity study. | No reservation | No reservation | No nutritional assessment has been done, because the applicant considers that composition equivalence has been demonstrated between maize MZHG0JG and conventional maize. | |||||||||||||||||
| Soybean | DAS-44406-6 | Dow AgroSciences LLC | EFSA-GMO-NL-2012-106 (Regulation (EC) No 1829/2003) |
3/21/2017 | Positive opinion | No reservation | No reservation | The compositional analysis identified no differences requiring further assessment, except for an increase (up to 31%) in lectin activity in soybean DAS-44406-6. Such increase is unlikely to raise additional concerns for food/feed safety and nutrition of soybean DAS-44406-6 as compared to its conventional counterpart and non-GM reference varieties. | No reservation | No reservation | No reservation | 7/7/2017 | Negative opinion | No reservation | Reservations | The additional data did not make it possible to demonstrate the equivalence of composition between soybean DAS-44406-6 and non-genetically modified soybeans, in particular for the lectin content of the seeds. | Reservations | 90-day study: the additional data do not allow to lift the reservations previously expressed regarding the single dose used and the lack of power calculation. | No reservation | No reservation | |||||||||||||||||
| Soybean | DAS-44406-6 | Dow AgroSciences LLC | EFSA-GMO-NL-2012-106 (Regulation (EC) No 1829/2003) |
- | - | 7/8/2013 | Negative opinion | No reservation | Reservations | For the seed, the chemical composition of soybean DAS-44406-6 is not equivalent to that of the control soybean. The statistical analysis concludes that the lectin content in soybean DAS-44406-6 is not equivalent to that of the control soybean and the non-transgenic varieties analysed. | Reservations | 90-day study: in view of the results of the compositional comparative analysis, which concluded that the lectin contents were not equivalent, the origin of the haematological changes should be argued. Furthermore, this study, implemented after the recommendations of Anses and EFSA published in 2011, does not take into account the evaluation of the statistical power of the test. The Anses report indicates that the power of the difference tests of these studies may in some cases be insufficient with 12 rats per group. Information regarding the herbicide treatments applied to the plants from which the tested meals were produced will have to be provided. | No reservation | No reservation | |||||||||||||||||||||||
| Maize | All sub-combinations of MON89034 x 1507 x MON88017 x 59122 independently of their origin | Dow AgroSciences and Monsanto | EFSA-GMO-CZ-2008-62 (Regulation (EC) No 1829/2003) |
10/14/2011 | Positive opinion | No reservation | No reservation | The EFSA GMO Panel considers it unlikely that interactions between the single maize events in all possible sub-combinations will occur that may impact on the food and feed safety and the nutritional properties of the whole food and feed. | No reservation | The EFSA GMO Panel considers it unlikely that interactions between the single maize events in all possible sub-combinations will occur that may impact on the food and feed safety and the nutritional properties of the whole food and feed. | - | - | |||||||||||||||||||||||||
| Maize | MON89034 x 1507 x MON88017 x 59122 and all sub-combinations of the individual events as present in its segregating progeny | Dow AgroSciences and Monsanto | EFSA-GMO-CZ-2008-62 (Regulation (EC) No 1829/2003) |
9/27/2010 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 6/2/2009 | Negative opinion | No reservation | No reservation | Reservations | 90-day study performed on MON89034: no convincing explanation regarding the origin of the bladder stones in the female rats fed the high dose of MON89034. No 90-day study with maize MON89034 x 1507 x MON88017 x 59122. | 90-day study performed on MON89034: historical data from 70 studies performed between 1999 and 2006 have been provided but they were not sufficient to conclude to the absence of link between the oral administration of maize MON89034 and the occurrence of bladder stones in female rats fed the high dose of MON89034. | No reservation | No reservation | Recombinant proteins have not been quantified in grains and diets used in the feeding study. A pathological event induced an unusual mortality rate (5 - 10%) affecting indifferently the chickens fed the GM and control maize. | |||||||||||||||||
| Soybean | 356043 | Pioneer | EFSA-GMO-UK-2007-43 (Regulation (EC) No 1829/2003) |
7/26/2011 | Positive opinion | No reservation | No reservation | No differences were identified between 356043 soybean and its conventional counterpart, except for the newly expressed proteins, for higher levels of the acetylated amino acids N-acetylaspartate (NAA) and N-acetylglutamate (NAG), and the odd-chain fatty acids heptadecanoic, heptadecenoic and heptadecadienoic acid in grains from 356043 soybean. The levels of these acetylated amino acids and odd chain fatty acids fall outside the natural ranges observed for other commercial non-GM soybean varieties. The observed differences are further evaluated in the Food/Feed safety assessment (section 5). | No reservation | No reservation | No reservation | 11/7/2011 | Positive opinion | No reservation | Consumer exposure to C17:0, C17:1 and C17:2 fatty acids showed equivalence to natural food products (butter, cheese, beef, pork). Potential toxicity of NAA and NAG has been reviewed and a literature study has been provided by the applicant. Evaluation of consumer exposure to NAA and NAG showed margin of exposure of 230 for NAG and 590 for NAA, indicating consumer safety. | No reservation | A 90-day study was provided and assessed. The use of a small number of animals (12 rats of each sex per group) increases the risk of having insufficient power for statistical tests. | ||||||||||||||||||||
| Soybean | 356043 | Pioneer | EFSA-GMO-UK-2007-43 (Regulation (EC) No 1829/2003) |
- | - | 12/21/2007 | Negative opinion | No reservation | Reservations | It is not possible to conclude to the substantial equivalence between soybean 356043 and its control because of significant higher levels in two fatty acids (heptadecanoic and heptadecenoic) and to very high levels in acetylated amino acids N-acetyl glutamate (NAG) et N-acetyl aspartate (NAA) resulting from the presence of the gat4601 transgene. | Reservations | Absence of a repeated-dose 90-day oral toxicity study on whole food/feed in rodents. | No reservation | No reservation | |||||||||||||||||||||||
| Cotton | 281-24-236 x 3006-210-23 | Dow AgroSciences | EFSA-GMO-NL-2005-16 (Regulation (EC) No 1829/2003) |
6/15/2010 | Positive opinion | No reservation | No reservation | A number of statistically significant differences were observed in derived products obtained through seed processing of cotton 281-24-236 x 3006-210-23 and its single events when compared to their conventional counterpart. The EFSA GMO Panel did not consider these differences being biologically relevant, because they were inconsistent (i.e. not in each year and/or location), and mostly within the background ranges. | No reservation | The EFSA GMO Panel has considered the outcomes of a 90-day rat feeding study even if according to the EFSA GMO Panel’s Guidance Document (EFSA, 2006a), animal safety studies with the whole food/feed are not necessary for this application. | No reservation | No reservation | 2/8/2011 | Positive opinion | No reservation | The applicant didn't provide a 90-day toxicity study in rodents but such a study, performed with diets containing 10% of cotton meals, has been published in a peer-reviewed journal. This publication doesn't reveal adverse effects. | No reservation | The analysis of the complementary data provided by the applicant regarding the feeding study allows to conclude that the consumption of the meals by the chickens doesn't reveal nutritional differences between those derived from cotton 281-24-236 x 3006-210-23 and those derived from the controls (near isogenic and conventional commercial varieties). | |||||||||||||||||||
| Cotton | 281-24-236 x 3006-210-23 | Dow AgroSciences | EFSA-GMO-NL-2005-16 (Regulation (EC) No 1829/2003) |
- | - | 10/13/2005 | Reserved opinion | No reservation | No reservation | No reservation | No reservation | Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | Reservations | It would be desirable to have access to the individual data and the corresponding statistical analysis for the feeding study in chickens. | Regarding the oil intended for human consumption, Afssa considers that the oil produced from cotton 281-24-236/3006-210-23 is as safe as the oil produced from a non genetically modified cotton. | ||||||||||||||||||||||
| Maize | 98140 | Pioneer Overseas Corporation | EFSA-GMO-UK-2008-53 (Regulation (EC) No 1829/2003) |
4/16/2013 | Inconclusive opinion | No reservation | Inconclusive | The minimum standards for the design of field trials, set out in the EFSA GMO Panel guidance document, were not met. | Inconclusive | In the absence of an appropriately performed comparative assessment, the Panel is not in a position to conclude on the compositional, agronomic and phenotypic characteristics of maize 98140 compared with conventional maize. The safety assessment could therefore not be completed, and has focused mainly on the newly expressed proteins and on specific metabolites resulting from the acetylase activity of the GAT4621 protein. The EFSA GMO Panel cannot conclude on the safety of the newly expressed GAT4621 protein, because the 28-day oral toxicity study was not performed in accordance with the respective OECD Guideline (relevant parameters, i.e. haematology and coagulation, were not analysed). | No safety concerns were identified for the newly expressed protein ZM-HRA. A repeated-dose 90-day oral toxicity study in rats was considered even if the use of a negative segregant as the sole control material has limitations for the safety assessment of food/feed from GM plants. | Inconclusive | Since no reliable information is available from the comparative analysis and in light of its relevance for the identification of possible unintended effects, the EFSA GMO Panel cannot conclude on the allergenicity of the whole GM plant. | The EFSA GMO Panel considers that there are no indications that the newly expressed GAT4621 and ZM-HRA proteins in maize 98140 may be allergenic in the intended conditions of exposure. | No reservation | A 42-day feeding study using broiler chickens was considered even if the use of a negative segregant as the sole control material has limitations for the safety assessment of food/feed from GM plants. | 2/5/2009 | Negative opinion | No reservation | Reservations | It is not possible to conclude to the substantial equivalence between maize 98140 and the controls. Given the acetyl-transferase activity on glyphosate, resulting from the presence of the gat4621 gene, the concentrations of N-acetyl glyphosate and of aminomethylphosphonic acid (AMPA) and N-acetyl AMPA should have been measured in maize 98140. | Reservations | Given the increased concentrations of NAA and NAG in maize 98140 as compared to the controls, the data of the toxicological assessment of these compounds, some of which are available in the literature, should have been provided, as well as the assessment of the potential effects on human health of these acetylated amino acids resulting from the consumption of products derived from maize 98140 as compared to other food products containing high concentrations of these compounds. Regarding the 90-day toxicity study in rats, the applicant should give more information to explain the increased level of alkaline phosphatase in the animals fed diets containing maize 98140 and provide the historical data to which he refers. The exposure rates of the animals fed with a diet containing maize 98140 should have been calculated, regarding the GAT4621 and ZM-HRA proteins, NAA and NAG, as well as the NOAELs that can be deduced from these data. The safety and exposure margins for each of these products should have been also calculated. | No reservation | Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | No reservation | |||||||||||
| Maize | Bt11 x MIR162 x 1507 x GA21 and three subcombinations independently of their origin | Syngenta | EFSA-GMO-DE-2010-86 (Regulation (EC) No 1829/2003) |
7/11/2018 | Positive opinion | No reservation | The risk assessment for the three subcombinations has been done without specific data and on the basis of assumptions. A minority opinion was expressed by a GMO Panel member. | No reservation | No reservation | No reservation | No reservation | 9/13/2012 | Negative opinion | No reservation | Reservations | The number of sites is not sufficient. The study was carried out without commercial varieties. No statistical test of equivalence. | No reservation | No reservation | Reservations | No feeding study. | |||||||||||||||||
| Maize | MON87460 | Monsanto | EFSA-GMO-NL-2009-70 (Regulation (EC) No 1829/2003) |
11/15/2012 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 4/20/2010 | Negative opinion | No reservation | No reservation | Reservations | The dose used for the safety assessment of the CspB protein is insufficient (OECD test n°420). | A new study should be submitted. | No reservation | No reservation | ||||||||||||||||||
| Cotton | 281-24-236 x 3006-210-23 x MON88913 | Dow Agrosciences LLC | EFSA-GMO-NL-2009-68 (Regulation (EC) No 1829/2003) |
4/19/2016 | Positive opinion | No reservation | No reservation | Some agronomic and phenotypic characteristics are different, but no safety concern. | No reservation | No substantial modifications in the composition of the derived food/feed, no indication of possible unintended effects and no interactions were identified, so no animal studies are required. | No reservation | No potential increase of allergenicity. | No reservation | No substantial modifications in the composition of the derived food/feed, no indication of possible unintended effects and no interactions were identified, so no animal studies are required. | 11/28/2013 | Negative opinion | No reservation | Reservations | No significant differences were observed, but the experimental design was inadequate. | Reservations | Since equivalence is not demonstrated, a 90-day study should be provided. The absence of interaction between the newly expressed proteins is not demonstrated. | No reservation | Reservations | The applicant considered that equivalence was demonstrated and therefore, he didn't provide a poultry trial. But the equivalence is not demonstrated. | |||||||||||||
| Maize | MON87427 x MON89034 x 1507 x MON88017 x 59122 and subcombinations independently of their origin | Monsanto Company | EFSA-GMO-BE-2013-118 (Regulation (EC) No 1829/2003) |
8/1/2017 | Positive opinion | No reservation | The only foreseen interactions at the biological level are between the Cry proteins in susceptible insects. | No reservation | Except for thiamine, none of the differences identified between maize MON87427 x MON89034 x 1507 x MON88017 x 59122 and the non-GM comparator in forage and grain composition and agronomic and phenotypic characteristics needs further assessment regarding food and feed safety. The nutritional impact of the reduced thiamine levels in maize MON87427 x MON89034 x 1507 x MON88017 x 59122 is further discussed in Section 3.3.3.5. | No reservation | No safety concerns to human and animal health related to the newly expressed proteins. Equivalence is demonstrated, so no animal studies are required. | No reservation | No reservation | 9/1/2015 | Negative opinion | No reservation | No reservation | No data for derived products. | Reservations | No previous reservation for maize 1507, MON88017, 59122 and MON87427, but previous reservations for MON89034. The absence of interaction between the newly expressed proteins is not demonstrated. | No reservation | No reservation | Equivalence is assumed since the composition is similar to that of conventional maize. Therefore, no animal trial was performed. But animal trials could help for the characterisation of potential interactions. | ||||||||||||||
| Maize | MON88017 | Monsanto | EFSA-GMO-CZ-2008-54 (Regulation (EC) No 1829/2003) |
11/10/2011 | Positive opinion | No reservation | No reservation | Statistically significant lower vitamin B1 content, in the range of that of conventional maize. Some phenotypic characteristics were different, but considered not biologically relevant. | No reservation | The 90-day study gave no indication of adverse effect. | No reservation | No potential increased allergenicity of maize MON88017. | No reservation | Poultry trial: significant differences for some parameters but in the biological range of the commercial varieties. | 12/5/2008 | Positive opinion | No reservation | The reservations expressed in the previous opinion are lifted. | No reservation | A new study was provided, the results of which confirm the previous ones and allow to conclude to the compositional equivalence of the grains of maize MON88017 and those of the control. | No reservation | The updated bioinformatic analyses (2008) confirm the previous conclusions regarding the CP4EPSPS and Cry3Bb1 proteins. | No reservation | The updated bioinformatic analyses (2008) confirm the previous conclusions regarding the CP4EPSPS and Cry3Bb1 proteins. These data are not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | No reservation | ||||||||||||
| Maize | MON88017 | Monsanto | EFSA-GMO-CZ-2008-54 (Regulation (EC) No 1829/2003) |
- | - | 4/4/2007 | Negative opinion | Reservations | Information is needed to know if the integration of event MON88017 has occurred in a functional region of the maize genome. | No reservation | No reservation | No reservation | Data not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | Reservations | No information regarding the presence of the CP4EPSPS protein in the diets. | ||||||||||||||||||||||
| Rapeseed | Ms8, Rf3 and Ms8 x Rf3 | Bayer | EFSA-GMO-BE-2010-81 (Regulation (EC) No 1829/2003) |
10/31/2012 | Positive opinion | No reservation | No reservation | No reservation | The EFSA GMO Panel is of the opinion that no additional animal safety studies are required. | No reservation | No reservation | Poultry trial: oilseed rape Ms8 × Rf3 is as nutritious as a commercial non-GM oilseed rape. | 1/5/2012 | Positive opinion | No reservation | Updated bioinformatic analyses (2011). | No reservation | The results of a new field trial were provided. Although the results of the statistical analysis are not clearly presented, these data confirm the compositional equivalence of the grains of rapeseed MS8, Rf3 and Ms8 x Rf3 with the control, in particular for the measured antinutritional compounds. | No reservation | Updated bioinformatic analyses (2011). | No reservation | Updated bioinformatic analyses (2011). | |||||||||||||||
| Rapeseed | MS11 | Bayer CropScience | EFSA-GMO-BE-2016-138 (Regulation (EC) No 1829/2003) |
5/14/2020 | Inconclusive opinion | No reservation | Inconclusive | Because of the lack of an appropriate data set, the GMO Panel is not in the position to conclude on the compositional analysis and can therefore not complete the comparative analysis. | Under the specific theoretical cultivation scenarios considered, the GMO Panel concludes that none of the differences identified in the agronomic and phenotypic characteristics tested between oilseed rape MS11 and its conventional counterpart needs further assessment. | Inconclusive | Owing to the incompleteness of the compositional analysis, the toxicological, allergenicity and nutritional assessment of oilseed rape MS11 cannot be completed. | The GMO Panel is not aware of any new information that would change previous conclusion on the safety of PAT/bar proteins and does not identify concerns regarding the potential toxicity of the Barnase, Barstar as expressed in this oilseed rape MS11. Based on scientific knowledge, no synergistic or antagonistic interactions raising food/feed safety concerns exist between the PAT/bar and the Barnase or Barstar proteins and their complex. Since the GMO Panel is not in the position to conclude on comparative analysis, is not possible to establish whether the 90-day study on Ms11 should be conducted according to a hypothesis-driven design or not (EFSA, 2014); therefore, the GMO Panel did not assess the 90-day study provided in the context of this application. | Inconclusive | Owing to the incompleteness of the compositional analysis, the toxicological, allergenicity and nutritional assessment of oilseed rape MS11 cannot be completed. | In the context of this application, the GMO Panel considers that there are no indications that the newly expressed Barnase, Barstar and/or PAT/bar proteins in oilseed rape MS11 may be allergenic. | Inconclusive | Owing to the incompleteness of the compositional analysis, the toxicological, allergenicity and nutritional assessment of oilseed rape MS11 cannot be completed. | 6/19/2017 | Negative opinion | No reservation | The elements provided don't show that this rapeseed is male sterile. Additionally, it would be useful that the applicant perform a transcriptional study of the region situated in 3' of the insert, to check if the expression of the ORF located there is changed as a result of the insertion of the T-DNA. | No reservation | Reservations | The applicant must provide the results and associated references of all the acute studies performed on the newly expressed proteins in rapeseed Ms11. Regarding the 28-day studies performed on the Barnase and Barstar proteins, it is not possible to conclude on the biological significance of the variations observed for the thymus and the seminal vesicles weights in the absence of the historical data of the investigation centre. If 28-day studies have been performed on the PAT protein, the results and associated references will have to be mentioned in the application. Regarding the 90-day study: the raw data under electronic format and calculation programs are not provided, the applicant used only one dose of 15%, referring to the EFSA guidance document (2014) to explain this choice, but the reference value given in this document is 25%, and the security of the oil is not documented since the study was performed with defatted meal. | The 28-day study performed with a combination of the Barnase and Barstar proteins doesn't reveal statistically significant differences with the control. | No reservation | No reservation | Although the applicant states that feeding studies are not necessary since the equivalence is demonstrated, he provides a poultry study and a catfish study which are not cited in the main text. | |||||||||
| Maize | 1507 x 59122 x MON810 x NK603 and subcombinations | Pioneer | EFSA-GMO-NL-2011-92 (Regulation (EC) No 1829/2003) |
11/28/2017 | Positive opinion | No reservation | Data on the levels of the newly expressed proteins were provided for 4 out of the 10 combinations not previously assessed. | No reservation | No reservation | Based on the known biological function of the newly expressed proteins, the only foreseeable interactions at the biological level are between the Cry proteins in susceptible insects. | No reservation | No reservation | 4/19/2012 | Negative opinion | No reservation | Reservations | The experimental design of the trial is not in line with EFSA recommendations: the number of sites is lower and no commercial varieties were included. | No reservation | No reservation | The updated bioinformatic analyses confirm the previous conclusions regarding the newly expressed proteins. These data are not sufficient to exclude an allergenic potential with certainty, but based on current scientific knowledge, such a certainty could not be achieved for any protein. | No reservation | ||||||||||||||||
| Maize | MON89034 x MON88017 | Monsanto | EFSA-GMO-NL-2007-39 (Regulation (EC) No 1829/2003) |
5/27/2010 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 11/20/2007 | Negative opinion | No reservation | No reservation | Reservations | Absence of a repeated-dose 90-day oral toxicity study on whole food/feed in rodents. | 90-day study performed on MON89034: no further explanation for renal histological changes. | No reservation | No reservation | ||||||||||||||||||
| Maize | NK603 x T25 | Monsanto | EFSA-GMO-NL-2010-80 (Regulation (EC) No 1829/2003) |
7/15/2015 | Positive opinion | No reservation | No reservation | No reservation | No reservation | No reservation | 10/7/2015 | Positive opinion | No reservation | Updated bioinformatic analyses. | No reservation | No reservation | No reservation for the 90-day toxicity study on maize T25 (provided in an other application). | No reservation | Updated bioinformatic analyses. | No reservation | |||||||||||||||||
| Maize | NK603 x T25 | Monsanto | EFSA-GMO-NL-2010-80 (Regulation (EC) No 1829/2003) |
- | - | 1/6/2011 | Negative opinion | No reservation | No reservation | Reservations | Absence of a repeated-dose 90-day oral toxicity study on whole food/feed in rodents for T25. | No reservation | No reservation | ||||||||||||||||||||||||
| Maize | Subcombination Bt11 x 1507 x GA21 related to the application EFSA-GMO-DE-2011-99 | Syngenta | EFSA-GMO-DE-2011-99 (Regulation (EC) No 1829/2003) |
12/19/2017 | Positive opinion | No reservation | No reservation | No reservation | No reservation | Information on the levels of the newly expressed proteins in subcombination Bt11 x 1507 x GA21 were provided. | No reservation | - | - | ||||||||||||||||||||||||
| Maize | Bt11 x 59122 x MIR604 x 1507 x GA21 and twenty subcombinations, which have not been authorised previously independently of their origin | Syngenta | EFSA-GMO-DE-2011-99 (Regulation (EC) No 1829/2003) |
8/26/2016 | Inconclusive opinion | No reservation | No specific data were available for any of the 20 subcombinations included in the scope of this application but 5 of these subcombinations have been previously assessed. | No reservation | No specific data were available for any of the 20 subcombinations included in the scope of this application but 5 of these subcombinations have been previously assessed. | No reservation | No specific data were available for any of the 20 subcombinations included in the scope of this application but 5 of these subcombinations have been previously assessed. | Inconclusive | At relatively high doses, the Bt proteins might act as adjuvants. Given the anticipated expression levels in the subcombinations that are similar to those measured in the singles, the five-event stack and the subcombinations already assessed, this scenario is considered extremely unlikely. However, to mitigate uncertainty, the EFSA GMO Panel has included a recommendation that, if any of the subcombinations were to be created via targeted breeding approaches and commercialised in the future, the applicant should provide relevant information including expression levels of the newly expressed proteins. | No specific data were available for any of the 20 subcombinations included in the scope of this application but 5 of these subcombinations have been previously assessed. | No reservation | No specific data were available for any of the 20 subcombinations included in the scope of this application but 5 of these subcombinations have been previously assessed. | 10/5/2016 | Negative opinion | No reservation | Inconclusive | The compositional equivalence between maize Bt11 x 59122 x MIR604 x 1507 x GA21 and conventional maize varieties is not demonstrated. | Reservations | Since equivalence is not demonstrated, a 90-day study should be provided. The absence of interaction between the newly expressed proteins is not argued enough. | No reservation | No reservation | It is not indicated whether maize Bt11 x 59122 x MIR604 x 1507 x GA21 used in this study has been treated with glyphosate and glufosinate. | |||||||||||
| Maize | MON89034 x 1507 x NK603 x DAS-40278-9 and subcombinations independently of their origin | Dow AgroSciences | EFSA-GMO-NL-2013-112 (Regulation (EC) No 1829/2003) |
1/16/2019 | Positive opinion | No reservation | Based on the known biological function of the newly expressed proteins, the only foreseen interactions at the biological level are between the Cry proteins in susceptible insects. For the six subcombinations included in the scope of this application for which no experimental data have been provided, the GMO Panel has assessed the possibility of interactions between the events and concludes that these combinations would not raise safety concerns. | No reservation | The GMO Panel concludes that none of the differences identified in forage and grain composition between the four-event stack maize, its non-GM comparator and the non-GM commercial reference varieties needs further assessment regarding food and feed safety, except for the changes in levels of cystine, isoleucine, phenylalanine, raffinose, manganese and b-carotene in grain and in levels of total fat in forage, which are further assessed in Section 3.3.3. For the six subcombinations included in the scope of this application for which no experimental data have been provided, the GMO Panel has assessed the possibility of interactions between the events and concludes that these combinations would not raise safety concerns. | No reservation | Based on the outcome of the studies considered in the molecular characterisation and comparative analysis, no substantial modifications of toxicological relevance in the composition of the four-event stack maize, and no indication of possible unintended effects relevant to food and feed safety have been identified. Therefore, animal studies on food and feed from the four-event stack maize are not necessary. For the six subcombinations included in the scope of this application for which no experimental data have been provided, the GMO Panel has assessed the possibility of interactions between the events and concludes that these combinations would not raise safety concerns. | No reservation | No reservation | Based on the outcome of the studies considered in the molecular characterisation and comparative analysis, no substantial modifications of toxicological relevance in the composition of the four-event stack maize, and no indication of possible unintended effects relevant to food and feed safety have been identified. Therefore, animal studies on food and feed from the four-event stack maize are not necessary. For the six subcombinations included in the scope of this application for which no experimental data have been provided, the GMO Panel has assessed the possibility of interactions between the events and concludes that these combinations would not raise safety concerns. | 2/10/2017 | Negative opinion | No reservation | Reservations | The compositional equivalence between maize MON89034 x 1507 x NK603 x DAS-40278-9 and conventional maize varieties is not demonstrated. No analysis performed on derived products. | Reservations | Reservations regarding the 90-day study on MON89034, no 90-day study for DAS-40278-9 and no 90-day study on the stack. The argument provided by the applicant regarding the risk of interactions is insufficient. | No reservation | Reservations | No feeding study. | |||||||||||||
no reviews yet
Please Login to review.