169x Filetype PDF File size 0.13 MB Source: www.bu.edu
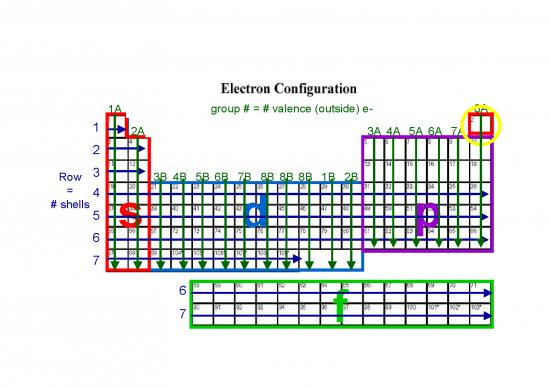
1A group # = # valence (outside) e- 8A
1 2A 3A 4A 5A 6A 7A
2
Row 3 3B 4B 5B 6B 7B 8B 8B 8B 1B 2B
= 4
# shells 5 s d p
6
7
6
7 f
Electron Configuration
1
1s
row # group #
shell # # valence e-
possibilities are 1-7 possibilities are:
7 rows s: 1 or 2
subshell p: 1-6
possibilities are d: 1-10
s, p, d, or f f: 1-14
4 subshells Total e- should equal
Atomic #
1
What element has an electron configuration of 1s ?
Practice:
Ask these questions every time you have to write an
electron configuration
Lithium:
1. find the element on the periodic table atomic # = 3
2. what is the period number? 2
3. how many shells? 2
4. what is the group number? 1
5. how many valence electrons? 1
s
6. what subshell(s) does Li have?
2 1
7. what is the electron configuration? 1s 2s
Practice:
Ask these questions every time you have to write an
electron configuration
Boron:
1. find the element on the periodic table atomic # = 5
2. what is the row #? 2
3. how many shells? 2
4. what is the group #? 3
5. how many valence electrons? 3
6. what subshell(s) does B have? p
2 2 1
7. what is the electron configuration? 1s 2s 2p
no reviews yet
Please Login to review.