275x Filetype PDF File size 0.38 MB Source: blog.pmt.education
Edexcel Chemistry IGCSE Advance Information
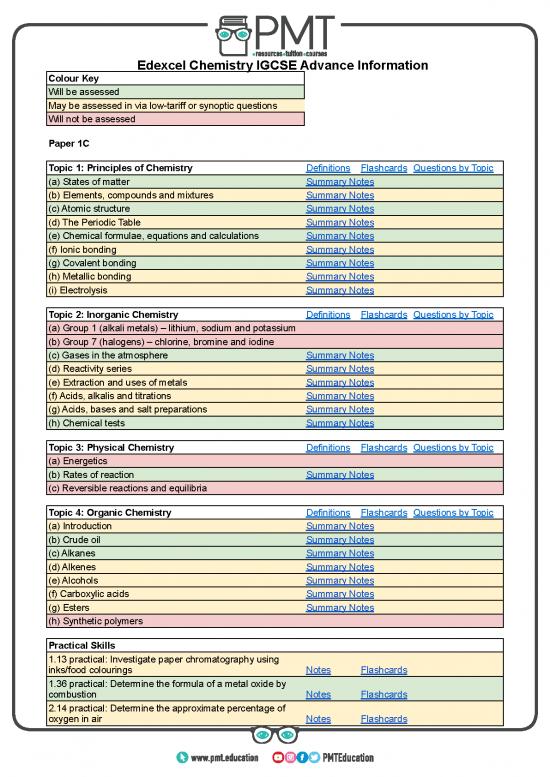
Colour Key
Will be assessed
May be assessed in via low-tariff or synoptic questions
Will not be assessed
Paper 1C
Topic 1: Principles of Chemistry Definitions Flashcards Questions by Topic
(a) States of matter Summary Notes
(b) Elements, compounds and mixtures Summary Notes
(c) Atomic structure Summary Notes
(d) The Periodic Table Summary Notes
(e) Chemical formulae, equations and calculations Summary Notes
(f) Ionic bonding Summary Notes
(g) Covalent bonding Summary Notes
(h) Metallic bonding Summary Notes
(i) Electrolysis Summary Notes
Topic 2: Inorganic Chemistry Definitions Flashcards Questions by Topic
(a) Group 1 (alkali metals) – lithium, sodium and potassium
(b) Group 7 (halogens) – chlorine, bromine and iodine
(c) Gases in the atmosphere Summary Notes
(d) Reactivity series Summary Notes
(e) Extraction and uses of metals Summary Notes
(f) Acids, alkalis and titrations Summary Notes
(g) Acids, bases and salt preparations Summary Notes
(h) Chemical tests Summary Notes
Topic 3: Physical Chemistry Definitions Flashcards Questions by Topic
(a) Energetics
(b) Rates of reaction Summary Notes
(c) Reversible reactions and equilibria
Topic 4: Organic Chemistry Definitions Flashcards Questions by Topic
(a) Introduction Summary Notes
(b) Crude oil Summary Notes
(c) Alkanes Summary Notes
(d) Alkenes Summary Notes
(e) Alcohols Summary Notes
(f) Carboxylic acids Summary Notes
(g) Esters Summary Notes
(h) Synthetic polymers
Practical Skills
1.13 practical: Investigate paper chromatography using
inks/food colourings Notes Flashcards
1.36 practical: Determine the formula of a metal oxide by
combustion Notes Flashcards
2.14 practical: Determine the approximate percentage of
oxygen in air Notes Flashcards
https://bit.ly/pmt-edu
2.21 practical: Investigate reactions between acids and
metals Notes Flashcards
2.42 practical: Prepare a sample of copper(II) sulfate
crystals Notes Flashcards
3.8 practical: Investigate temperature changes
3.15 practical: Investigate the effect of surface area on rate
of reaction Notes Flashcards
3.16 practical: Investigate the effect of different solids on
catalytic decomposition Notes Flashcards
Paper 2C
Topic 1: Principles of Chemistry Definitions Flashcards Questions by Topic
(a) States of matter
(b) Elements, compounds and mixtures
(c) Atomic structure
(d) The Periodic Table Summary Notes
(e) Chemical formulae, equations and calculations Summary Notes
(f) Ionic bonding Summary Notes
(g) Covalent bonding
(h) Metallic bonding Summary Notes
(i) Electrolysis Summary Notes
Topic 2: Inorganic Chemistry Definitions Flashcards Questions by Topic
(a) Group 1 (alkali metals) – lithium, sodium and potassium
(b) Group 7 (halogens) – chlorine, bromine and iodine Summary Notes
(c) Gases in the atmosphere Summary Notes
(d) Reactivity series
(e) Extraction and uses of metals Summary Notes
(f) Acids, alkalis and titrations Summary Notes
(g) Acids, bases and salt preparations Summary Notes
(h) Chemical tests Summary Notes
Topic 3: Physical Chemistry Definitions Flashcards Questions by Topic
(a) Energetics Summary Notes
(b) Rates of reaction
(c) Reversible reactions and equilibria Summary Notes
Topic 4: Organic Chemistry Definitions Flashcards Questions by Topic
(a) Introduction
(b) Crude oil Summary Notes
(c) Alkanes Summary Notes
(d) Alkenes
(e) Alcohols
(f) Carboxylic acids Summary Notes
(g) Esters Summary Notes
(h) Synthetic polymers Summary Notes
Practical Skills
1.7C practical: Investigate the solubility of a solid in water
1.13 practical: Investigate paper chromatography using
inks/food colourings
https://bit.ly/pmt-edu
1.36 practical: Determine the formula of a metal oxide by
combustion Notes Flashcards
1.60C practical: Investigate the electrolysis of aqueous
solutions Notes Flashcards
2.14 practical: Determine the approximate percentage of
oxygen in air Notes Flashcards
2.21 practical: Investigate reactions between acids and
metals
2.42 practical: Prepare a sample of copper(II) sulfate
crystals Notes Flashcards
2.43C practical: Prepare a sample of pure, dry lead(II)
sulfate Notes Flashcards
3.8 practical: Investigate temperature changes Notes Flashcards
3.15 practical: Investigate the effect of surface area on rate
of reaction
3.16 practical: Investigate the effect of different solids on
catalytic decomposition
4.43C practical: Prepare a sample of an ester such as ethyl
ethanoate Notes Flashcards
https://bit.ly/pmt-edu
no reviews yet
Please Login to review.