223x Filetype DOC File size 0.18 MB Source: www.asperbio.com
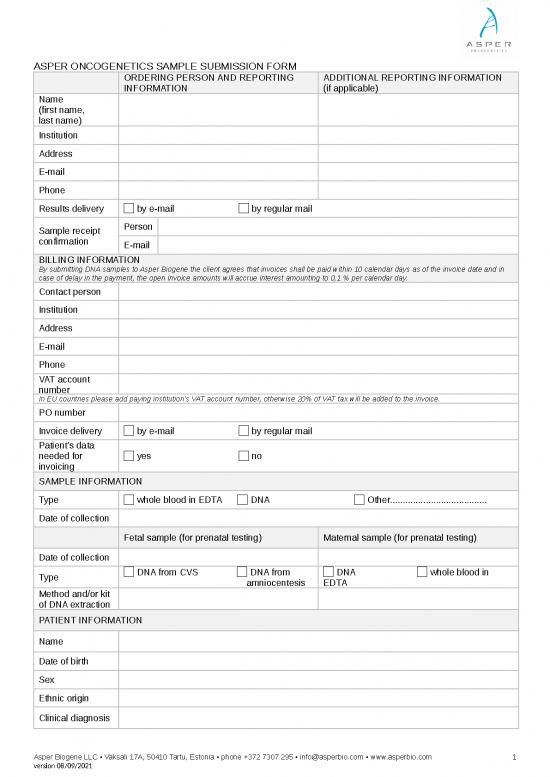
ASPER ONCOGENETICS SAMPLE SUBMISSION FORM
ORDERING PERSON AND REPORTING ADDITIONAL REPORTING INFORMATION
INFORMATION (if applicable)
Name
(first name,
last name)
Institution
Address
E-mail
Phone
Results delivery by e-mail by regular mail
Sample receipt Person
confirmation E-mail
BILLING INFORMATION
By submitting DNA samples to Asper Biogene the client agrees that invoices shall be paid within 10 calendar days as of the invoice date and in
case of delay in the payment, the open invoice amounts will accrue interest amounting to 0,1 % per calendar day.
Contact person
Institution
Address
E-mail
Phone
VAT account
number
In EU countries please add paying institution's VAT account number, otherwise 20% of VAT tax will be added to the invoice.
PO number
Invoice delivery by e-mail by regular mail
Patient’s data
needed for yes no
invoicing
SAMPLE INFORMATION
Type whole blood in EDTA DNA Other......................................
Date of collection
Fetal sample (for prenatal testing) Maternal sample (for prenatal testing)
Date of collection
Type DNA from CVS DNA from DNA whole blood in
amniocentesis EDTA
Method and/or kit
of DNA extraction
PATIENT INFORMATION
Name
Date of birth
Sex
Ethnic origin
Clinical diagnosis
Asper Biogene LLC • Vaksali 17A, 50410 Tartu, Estonia • phone +372 7307 295 • info@asperbio.com • www.asperbio.com 1
version 08/09/2021
ASPER ONCOGENETICS TESTS
NGS panel of genes with CNV
Sequencing of BRCA1, BRCA2 genes
Breast and Ovarian Cancer Del/dup analysis of BRCA1, BRCA2, CHEK2 genes by
MLPA
Sequencing + del/dup analysis of BRCA1, BRCA2 genes
by MLPA
Cancer Predisposition NGS panel of genes with CNV
Sequencing of APC gene
Familial Adenomatous Polyposis
Del/dup analysis of APC gene by MLPA
NGS panel of genes with CNV
Fanconi Anemia Del/dup analysis of FANCA, FANCB, FANCD2, PALB2
genes by MLPA
NGS panel of genes with CNV
Microsatellite instability
Sequencing of MLH1 gene
Lynch Syndrome Sequencing of MSH2 gene
Sequencing of MSH6 gene
Del/dup analysis of MLH1, MSH2 genes by MLPA
Del/dup analysis of MSH6 gene by MLPA
NGS panel of genes with CNV
Melanoma Del/dup analysis of CDK4, CDKN2A, CDKN2B, MITF
genes by MLPA
Sequencing of MUTYH gene
MUTYH-Associated Polyposis Targeted mutation analysis
Del/dup analysis of GREM1, MUTYH, SCG5 genes by
MLPA
Sequencing of NBN gene
Nijmegen Breakage Syndrome
Targeted mutation analysis
NGS panel of genes with CNV
Polyposis Syndromes Del/dup analysis of BMPR1A, PTEN, SMAD4, STK11
genes by MLPA
Prostate Cancer NGS panel of genes with CNV
Renal Cancer NGS panel of genes with CNV
Asper Biogene LLC • Vaksali 17A, 50410 Tartu, Estonia • phone +372 7307 295 • info@asperbio.com • www.asperbio.com 2
version 08/09/2021
ASPER ONCOGENETICS TESTS
NGS panel of genes with CNV
Thyroid Cancer Del/dup analysis of MEN1, SDHB, SDHC, SDHD genes by
MLPA
Von Hippel-Lindau Disease Sequencing of VHL gene
CUSTOM TEST
NGS panel of genes with CNV
Del/dup analysis by MLPA
Del/dup analysis of selected regions by
Chromosomal Microarray Analysis
Single gene sequencing
Single mutation analysis
PATIENT’S CLINICAL INFORMATION
Reason for referral
confirmation of clinical diagnosis testing of at-risk family members
cancer predisposition assessment risk assessment for adverse drug reactions
Age at the onset of symptoms………….............................
Patient´s clinical features
no symptoms
cancer,
location..............................................................................................................................................................
Previous genetic testing
not done
results:
.......................................................................................................................................................................................
.......................................................................................................................................................................................
Family history
unknown
diagnosis…………………………………………………………………………………………………………........................
specify the relation to the proband and age at diagnosis………………………………………………………………….....
.......................................................................................................................................................................................
Authorization to use remaining sample material and test results
Asper Biogene may use de-identified (without personal identifying information) remaining sample material and test re-
sults for quality improvements and/or scientific purposes.
I give my consent to use my de-identified sample material and test results as described above
I do not give my consent to use my de-identified sample material and test results as described above
Name of patient………………………………………………………………………………………………………………………
Patient’s signature……………………………………………………………………………………………………………………
Date……………………………………………………………………………………………………………………………………
Asper Biogene LLC • Vaksali 17A, 50410 Tartu, Estonia • phone +372 7307 295 • info@asperbio.com • www.asperbio.com 3
version 08/09/2021
Important: By sending samples and placing an order customer accepts Terms and Conditions and Privacy Policy of
Asper Biogene (see website for details).
Asper Biogene LLC • Vaksali 17A, 50410 Tartu, Estonia • phone +372 7307 295 • info@asperbio.com • www.asperbio.com 4
version 08/09/2021
no reviews yet
Please Login to review.